Best management guide
Chickpea production: northern region
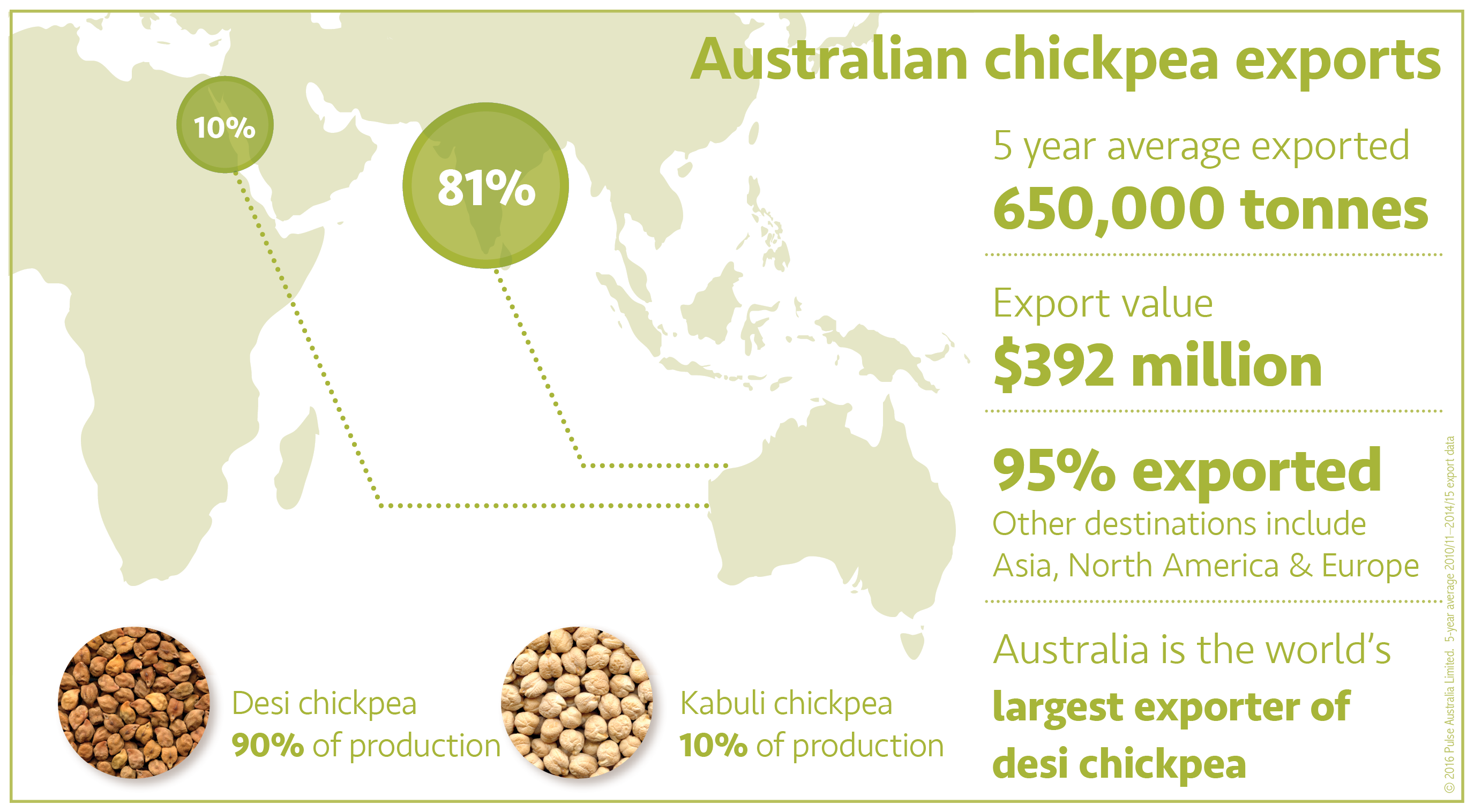
Australian industry
The first commercial chickpea (Cicer arietinum) crop in Australia was grown near Goondiwindi during the early 1970s. Since then chickpea has become an important crop in northern farming systems. The dedicated efforts of farmers, researchers and agronomists has ensured the industry’s continued success, particularly through the development and adoption of an effective management package that has minimised the threat of the potentially devastating disease, ascochyta blight.
There are two groups of chickpea – desi and kabuli – mainly distinguished by seed size, shape and colour. The two types have different production requirements, markets and end-uses. Most Australian chickpea (mainly desi type) production is in northern Australia, and nearly all of the grain is exported.
The main market for desi chickpea is India and Pakistan, and to Indian communities in other parts of the world (such as Britain and Western Canada). Buyers in India and Pakistan prefer larger, light coloured desi chickpea grain.
Types
Desi types are generally earlier maturing and higher yielding than the kabuli types, particularly the larger seeded kabulis.
Desi chickpea
- Desi types have small angular seeds, each weighing about 120 mg, that are wrinkled at the beak and the different varieties range in colour including brown, light brown, fawn, yellow, orange, black or green seed.
- The grain is normally dehulled and split to obtain dhal and then may be further processed to produce flour (besan). Buyers in India and the sub-continent favour desi chickpea over kabuli.
- There is an increasing use of large, whole seeded desi types in a range of food preparations in Bangladesh. A small premium has been paid for desi types (e.g. Kyabra) fitting this use.
- Desi chickpeas have traditionally made up about 90% to 95% of Australian production.
Kabuli chickpea
- Kabuli, sometimes called garbanzos, have larger, rounder seeds than desi types. Each seed weighs about 400 mg.
- They are white to cream in colour and are almost exclusively used whole. Buyers in the Mediterranean region prefer kabuli type chickpea.
- Within the kabuli types several market categories have emerged for Australian growers including the traditional large seeded kabuli markets, where large seed size (9 mm and above) is important to attract premium prices, a small seeded (7–8 mm) class than can be sold into bulk kabuli markets or graded to size to grade out the 8 mm class and a very small seeded class (less than 7 mm) than is sold into bulk kabuli markets.
Place in the rotation
Chickpea is a winter pulse crop well-suited to the northern grain production region. It is the most widely grown pulse crop in the region and is a permanent part of the cropping rotation on many farms.
| Advantages | Disadvantages |
|
|
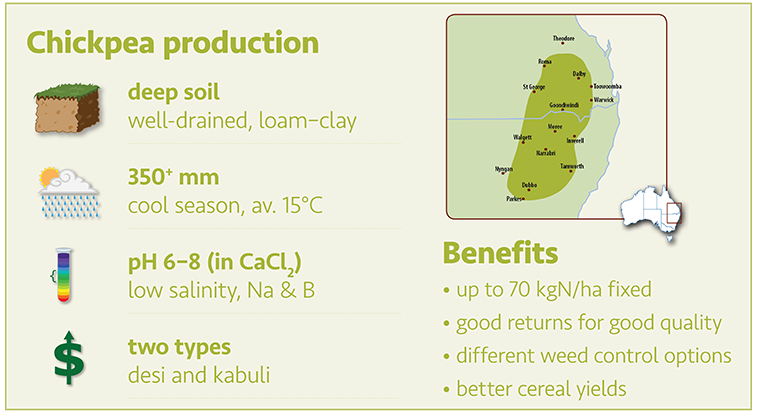
Chickpea prefer well drained loam to clay soils with a pH in the range of 6 to 8. They will not grow in light acid soils and areas prone to waterlogging should be avoided. Chickpea crops must be harvested close to the ground so stony paddocks or fields with uneven soil surface (e.g. gilgai) should be avoided.
Chickpea are susceptible to hostile sub-soils, with boron toxicity, sodicity and salinity causing patchiness in affected paddocks. Chickpea will not tolerate soils with any exchangeable aluminium present. Tolerance to sodicity in the root zone (to 90 cm) is less than 1% exchangeable sodium (ESP) on the surface and less than 5% ESP in the subsoil.
Broadleaf weeds and resistant grasses can cause major problems and a careful management strategy must be worked out well in advance. If possible, control weeds in the year prior to sowing chickpea or avoid paddocks with specific weeds that cannot be controlled by the available herbicides.
Foliar sprays of zinc and manganese may be needed if these deficiencies are identified.
Chickpea are not well suited to the lower rainfall, hotter areas, although the plants will set seed under warmer conditions where other pulse crops are likely to fail.
Cool wet conditions are more likely to stimulate foliar diseases and these can adversely affect seed set and yield.
Controlling ascochyta blight is a major consideration in chickpea. Variety choice, crop hygiene, fungicide choice and application timing are all important in an overall chickpea management strategy for ascochyta blight. Several varieties are now available that are resistant to ascochyta blight during the vegetative phases of the crop however all varieties remain susceptible to the disease during podding.
Provided ascochyta blight resistant varieties are used, delayed sowing is no longer necessary and strategic foliar fungicide use may only be required at podding. Susceptible varieties require fungicides to be applied regularly throughout the growing season.
Desi varieties should only be grown in areas where the annual rainfall is greater than 350 mm. Sowing is best carried out from early May to early June with early sowing recommended for the lower rainfall areas.
Kabuli varieties are later maturing and should only be grown in areas where the annual rainfall is over 450 mm.
For best results with chickpea, follow the recommendations in this guide wherever possible to minimise production risks and optimise the crop’s performance.
Keys to successful chickpea production
- Make chickpeas part of an integrated cropping system involving wheat, canola or barley. By taking a systems perspective and assess financial performance over several seasons you can estimate the true benefits of chickpeas in a rotation.
- Choose the right variety based on long term yield data for your region, maturity, disease resistance and the market opportunities for human consumption.
- Use good quality seed with high germination (80%+) and vigour that is free from seed-borne ascochyta and botrytis infection.
- Select and manage paddocks well in advance to control weeds and retain crop residues. Select paddocks with free draining soil of more neutral pH and low sodicity, salinity and boron toxicity. Herbicide residues need to be considered, as well as likely weed pressure and the ability to effectively control weeds before seeding and in-crop.
- Establish sufficient plants as recommended for the variety, situation and sowing time. Ideally ground cover should coincide with the time pod filling begins. Plant populations can range from 30–50 plants/m2 for rain-fed crops or higher if sown late.
- Sow on time. Sowing time is critical and regional recommendations should be followed. Late sowing reduces yield potential by lowering crop biomass, shortening the pod-filling period and increasing risks from moisture stress and high temperatures. Early sowing can lead to bulky crops, poor early pod set and greater disease risks.
- Ensure good nutrition. Nitrogen (N) fertiliser is unnecessary as the crop’s N requirement will be met through symbiotic fixing of atmospheric nitrogen, provided the seed is inoculated at sowing with the correct commercially available rhizobia inoculant. On phosphorus (P) deficient country, apply fertiliser at rates similar to or slightly less than those for wheat. On alkaline clay soils, fertilising with zinc may be warranted. On acid soils, molybdenum may be deficient and should be applied at or before sowing.
- Know the disease threats to chickpea and how to manage them for your district. No varieties are resistant to all fungal and virus diseases. The major risks are ascochyta blight, botrytis grey mould and plant viruses. The impact of fungal diseases on yield can be diminished through the strategic selection and use of fungicides and crop management. Maintain an isolation distances of more than 500 m from the previous year’s chickpea stubble, and eradicate volunteer plants over summer and autumn as these plants are a source aphids (potential plant virus vectors) and disease inoculum.
- Control insect pests such as aphids and helicoverpa. Controlling the ‘green bridge’ of weeds and volunteer crop plants over summer can reduce aphid populations and reduce the numbers that can infest the crop in the early growth stages. Monitor for helicoverpa (native budworm) caterpillars is essential during podding. Caterpillar feeding during podding will affect yield and potentially render the seed unsuitable for human consumption markets.
- Harvest on time, with a properly set-up header. Start harvesting when the seeds in the majority of pods ‘rattle’. Stems may not be completely dry at this stage. Pods will thresh easily to yield clean, whole seeds with a minimum of splits and cracks provided the header settings are correct.
- Have a marketing plan that includes plans for on-farm storage or delivery options off-header. Investigate forward contracting if storage, pools or warehousing is not an option.
- Optimise irrigation set-up and timing. Chickpeas respond well to irrigation in dry areas. Furrow irrigation is preferred over over-head irrigation. If necessary, pre-water then sow. To maximise yield potential, irrigate crops to produce maximum biomass while avoiding over-watering as chickpea crops will not tolerate waterlogging. Do not allow the plants to stress during flowering and pod-fill.
Plant physiology
Chickpea germination is hypogeal, with the cotyledons remaining below the soil surface. This enables it to emerge from sowing as deep as 15 cm. In arid regions, chickpea is sown deep as surface moisture is often inadequate to allow adequate crop establishment.
The chickpea plant is erect and freestanding, usually 15–60 cm in height, although well-grown plants may grow to 80 cm. The plants have a fibrous taproot system, a number of woody stems forming from the base, upper secondary branches and fine, frond-like leaves.
The node from which the first branch arises on the main stem above the soil is counted as node one. In chickpeas, alternate primary branches usually originate from nodes just above ground level (usually one to eight primary branches on the main stem, depending on growing conditions). A node is counted as developed when 6–15 leaflets have unfolded and flattened out.
Each leaflet has a thick covering of glandular hairs that secrete a strong acid (mostly malic acid), particularly during pod-set. The malic secretions from all vegetative surfaces of the plant seem to play a role in protecting the plant against pests such as red-legged earth mite, lucerne flea, aphids and pod borers. Similar substances are also secreted from the root system and can solubilise soil-bound phosphate and other nutrients. The acid also corrodes leather boots.
Chickpea plants can derive more than 70 per cent of their nitrogen requirement from symbiotic nitrogen fixation. Chickpea roots develop symbiotic nodules with the Rhizobium bacteria, capable of fixing atmospheric nitrogen. The plant provides carbohydrates for the bacteria in return for nitrogen fixed inside the nodules.
These nodules are visible within about a month of plant emergence, and eventually form slightly flattened, fan-like lobes. Practically all nodules are confined to the top 30 cm of soil and 90% are within the top 15 cm of the profile. When cut open, nodules actively fixing nitrogen have a pink centre. Nitrogen fixation is highly sensitive to waterlogging so it is essential that chickpea crops are grown on well aerated and drained soils.
Yields are best in areas with reliable winter rainfall for crop growth and mild spring conditions during seed filling. Chickpea is well suited to well-drained, non-acidic soils with medium to heavy clay texture.
Chickpeas are considered very indeterminate in their growth habit, i.e. their terminal bud is always vegetative and keeps growing, even after the plant switches to reproductive mode and flowering begins.
Temperature, day length and drought are the three major factors affecting flowering in chickpea. Temperature is generally more important than day length. Flowering and pod set in chickpea requires an average daily temperature of 15°C and cool wet conditions at flowering can adversely affect pod and seed set. Flowering is invariably delayed under low temperatures but more branching occurs.
Progress towards flowering is rapid during long days (17+ hours) and flowering is delayed but never prevented under short day (<17 hrs) conditions.
Chickpeas, unlike other cool season legumes, are very susceptible to cold conditions. This is particularly so at flowering and any advantage derived from early flowering is often negated by increased flower and pod abortion due to late frosts or cold snaps. Pods at a later stage of development are generally more resistant to frost than flowers and small pods, but may suffer some mottled darkening of the seed coat.
-
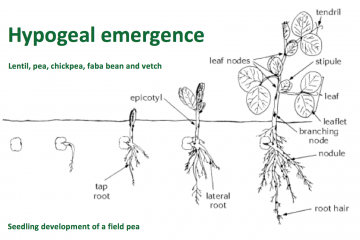
Hypogeal emergence of chickpea seedlings makes the plant less prone to environmental stress and damage in the early growth stages.
-

Chickpea plants have alternate leaves along the branch, with multiple leaflets on each leaf. Photo: G. Cumming
-

Desi chickpea varieties typically have pink flowers. Photo: Gordon Cumming.
-

Kabuli chickpeas typically have white flowers. Photo: Gordon Cumming.
-

Nodulated chickpea roots. The majority of nodules form on roots growing in the top 15 cm zone of the soil profile. Photo: G. Cumming
Variety selection
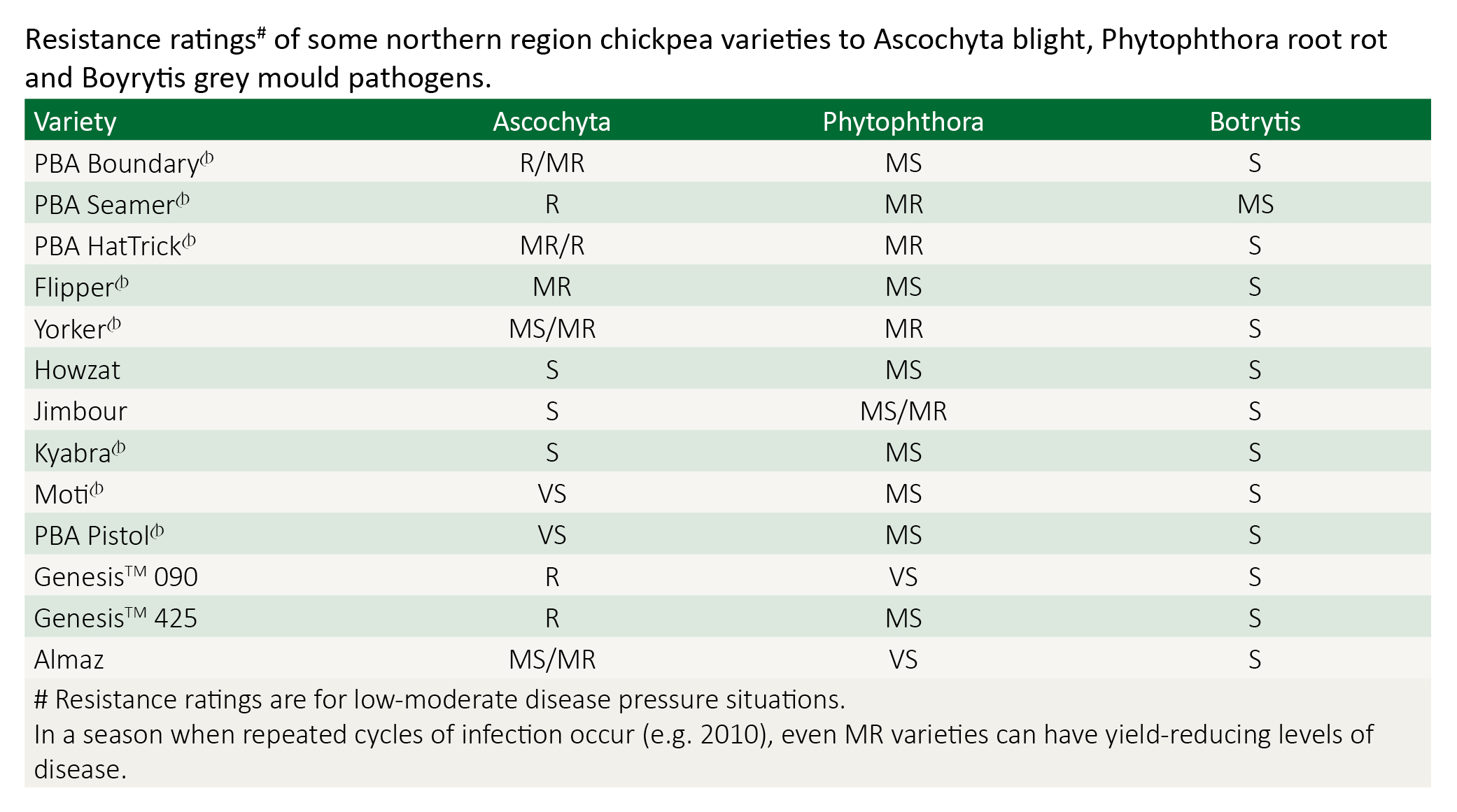
The availability of varieties resistant to ascochyta blight now provides growers with low disease risk options for growing chickpea in northern Australia. Ascochyta blight of chickpeas has been a widespread and devastating disease in all Australian production areas and unless resistant varieties are used, can be a major limitation to overcome when growing this crop.
Some ascochyta blight resistant varieties available to growers may have other agronomic, disease or marketability limitations and will not suit all areas or situations (e.g. PBA Boundary, which is susceptible to phytophthora root rot). When choosing varieties to grow, it is essential to consider their susceptibility to ascochyta blight and phytophthora root rot (PRR) along with yield potential, price potential, marketing opportunities, flowering cold tolerance, maturity timing, lodging resistance and other agronomic features relevant to your growing region.
When comparing yields between varieties, growers need to be aware that under high ascochyta blight pressure, varieties with moderate resistance or less are more likely to suffer greater yield losses than the resistant lines, even with regular applications of foliar fungicides.
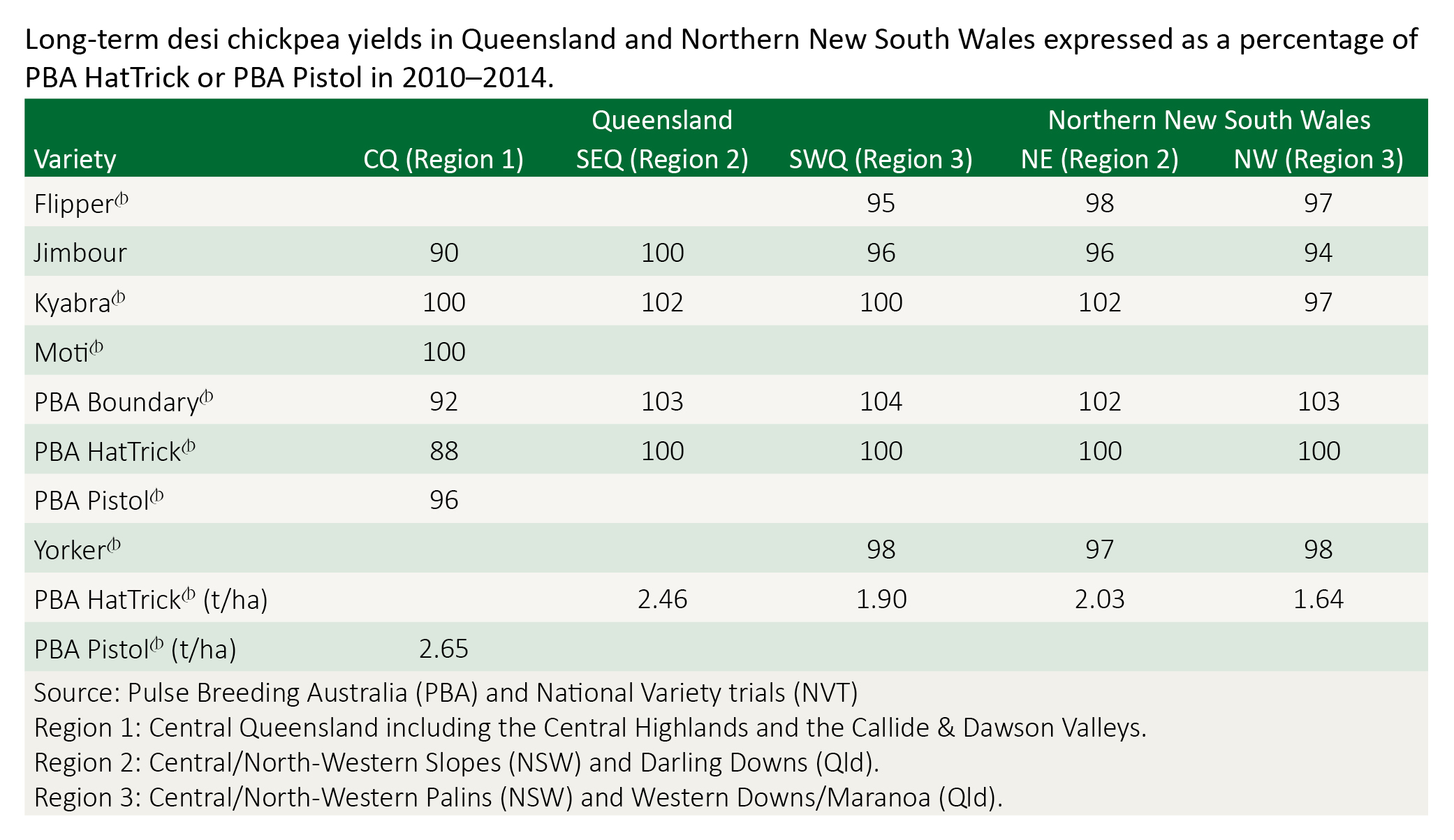
Pulse Breeding Australia (PBA) categorises chickpea production areas into five regions based on rainfall and geographic location. Regions 1, 2 and 3 fall within the northern cropping region. These regions cross state borders, and are target zones for national plant breeding and variety evaluation. Breeding trials and National Variety Trial (NVT) results help indicate specific adaptation even within a region.

There have been variety releases specific for central Queensland (PBA Pistol and Moti) and for southern Queensland and northern New South Wales (PBA HatTrick and PBA Boundary).
Desi chickpea varieties
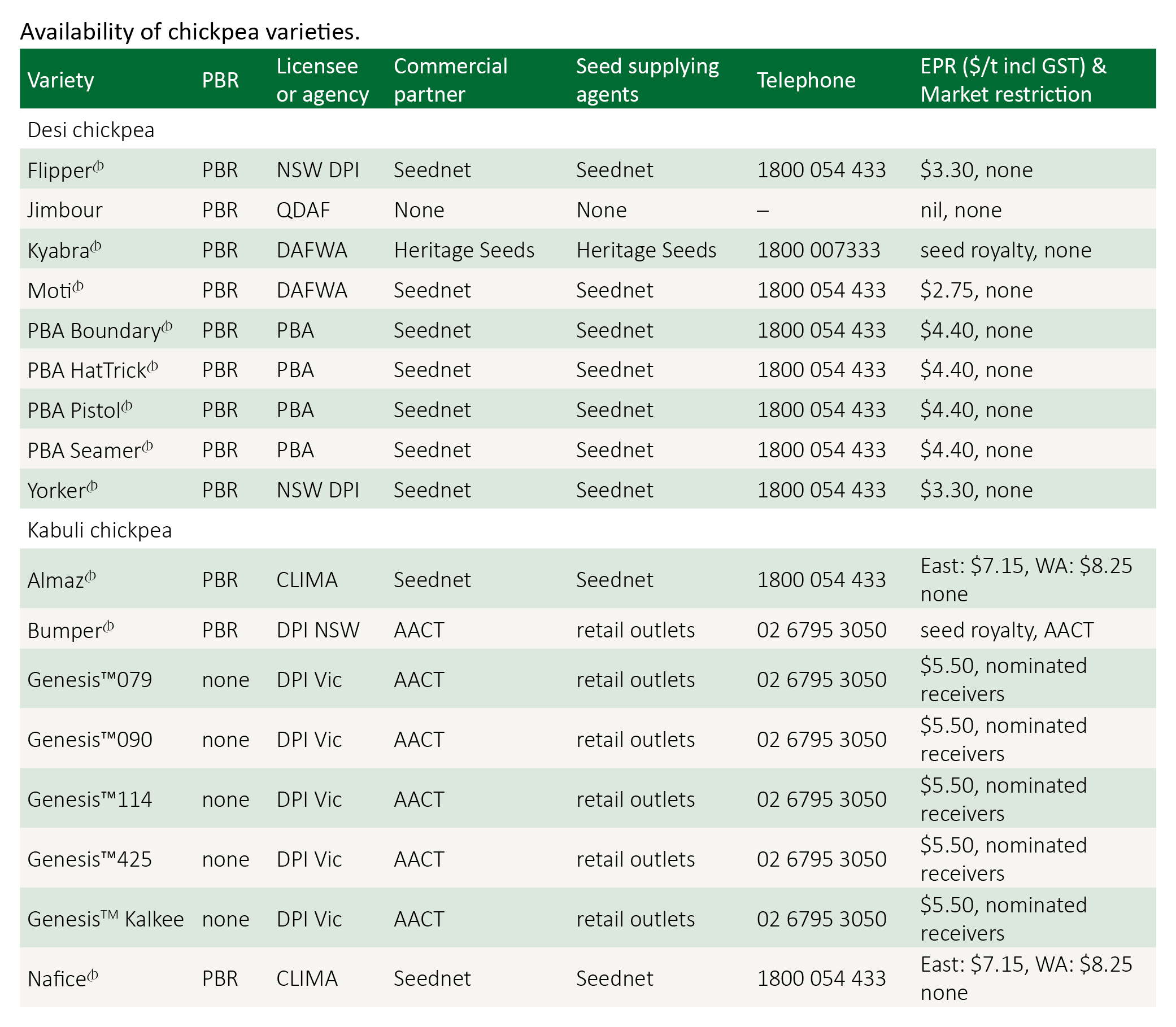
Kyabra was released for southern and central Queensland in 2005 where it was high yielding in those environments. It is highly susceptible to ascochyta blight but has excellent seed quality, and is often segregated to exploit this for marketability. Its grain is large, uniform and light in colour, making Kyabra well suited to the whole seed export market. Seed distribution is licensed to Heritage Seeds.
Area of Adaption: Zones 1, 2 and 3.
Moti was released specifically for central Queensland in 2003 by the Western Australia Department of Agriculture (DAWA). Moti rapidly reaches flowering and pod set and has offered exceptional performance from planting dates in May through to late June in CQ trials. Due to its early flowering care should be taken with early planting dates in areas that are prone to heavy frosts. Seed of PBA Boundary is available through Seednet.
Area of Adaption: Zone 1. Moti must not be grown south of Theodore/Rolleston due to its susceptibility to ascochyta blight. For more detailed information: Moti Variety Management Package
PBA Boundary was released from Pulse Breeding Australia in 2011 as a high-yielding, ascochyta blight moderately resistant (MR) desi chickpea variety suited to northern NSW and southern Queensland. It offers slightly increased ascochyta blight resistance over PBA HatTrick, but is susceptible to phytophthora root rot. PBA Boundary has a major yield advantage over existing varieties in both wetter (Region 2) and drier (Region 3) parts of the Northern Region. A reduced resistance to Phytophthora root rot means that PBA Boundary should not be grown in paddocks known to have a history of this disease or which are prone to waterlogging.
PBA Boundary is not recommended for Central Qld where yields are significantly lower than currently recommended varieties. An industry moratorium prevents the movement of seed from Regions 2 and 3 in northern Australia into Central Qld. Seed of PBA Boundary is available through Seednet.
Area of Adaption: Zones 2 and 3. For more detailed information: PBA Boundary Variety Management Package
PBA HatTrick was released from Pulse Breeding Australia in 2009 and is well suited to all current chickpea growing areas in northern NSW and southern Qld. It has quickly become the variety of choice in those areas because it was the first variety for northern Australia to combine moderate resistance to the two key disease problems in north-eastern Australia (ascochyta blight and phytophthora root rot) with high yield potential. PBA HatTrick has a tall erect plant type, is of mid-season maturity, has medium seed size with excellent milling quality and is well suited to the direct human consumption market.
PBA HatTrick is not recommended for Central Qld where yields are significantly lower than those of the established varieties. An industry moratorium prevents the movement of seed from Regions 2 and 3 in northern Australia into Central Qld. Seed of PBA HatTrick is available through Seednet.
Area of Adaption: Zones 2 and 3. For more detailed information: PBA HatTrick Variety Management Package
PBA Pistol was released from Pulse Breeding Australia on 2011 as a replacement for Moti in central Queensland, combining high yields with excellent agronomy and grain quality. It is well adapted to the shorter growing season of central Queensland and has consistently produced higher yields than the current commercial varieties in a diverse range of seasonal conditions.
PBA Pistol is taller, more resistant to lodging and offers improved harvestability compared to other current commercially available varieties. PBA Pistol must not be grown south of Theodore/Rolleston due to its susceptibility to ascochyta blight. Seed of PBA Pistol is available through Seednet.
Area of Adaption: Zone 1. For more detailed information: PBA Pistol Variety Management Package
PBA Seamer was released by Pulse Breeding Australia in 2016 as an improved desi chickpea for the northern region with the highest available Ascochyta blight resistance rating (rated R). It is broadly adapted from central NSW to central Queensland, with significantly higher grain yield than all current varieties in high disease years.
PBA Seamer has a semi-erect plant type with superior lodging resistance to PBA HatTrick and PBA Boundary. PBA Seamer has improved seed quality with larger seed size than PBA HatTrick and higher dhal milling yield than all current varieties in southern Queensland and northern NSW.
Area of Adaption: Zones 1, 2 and 3. For more detailed information: PBA Seamer Variety Management Package
Yorker is moderately resistant to phytophthora root rot and was released in 2005 by the national chickpea breeding program for the lower rainfall areas of north-central NSW. Yorker has intermediate resistance to ascochyta blight. Yorker has a medium to large, light coloured seed well suited to the whole seed export market. Seed of Yorker is obtained through Seednet.
Area of Adaption: Zones 2 and 3. For more detailed information: Yorker Variety Management Package

Kabuli chickpea varieties
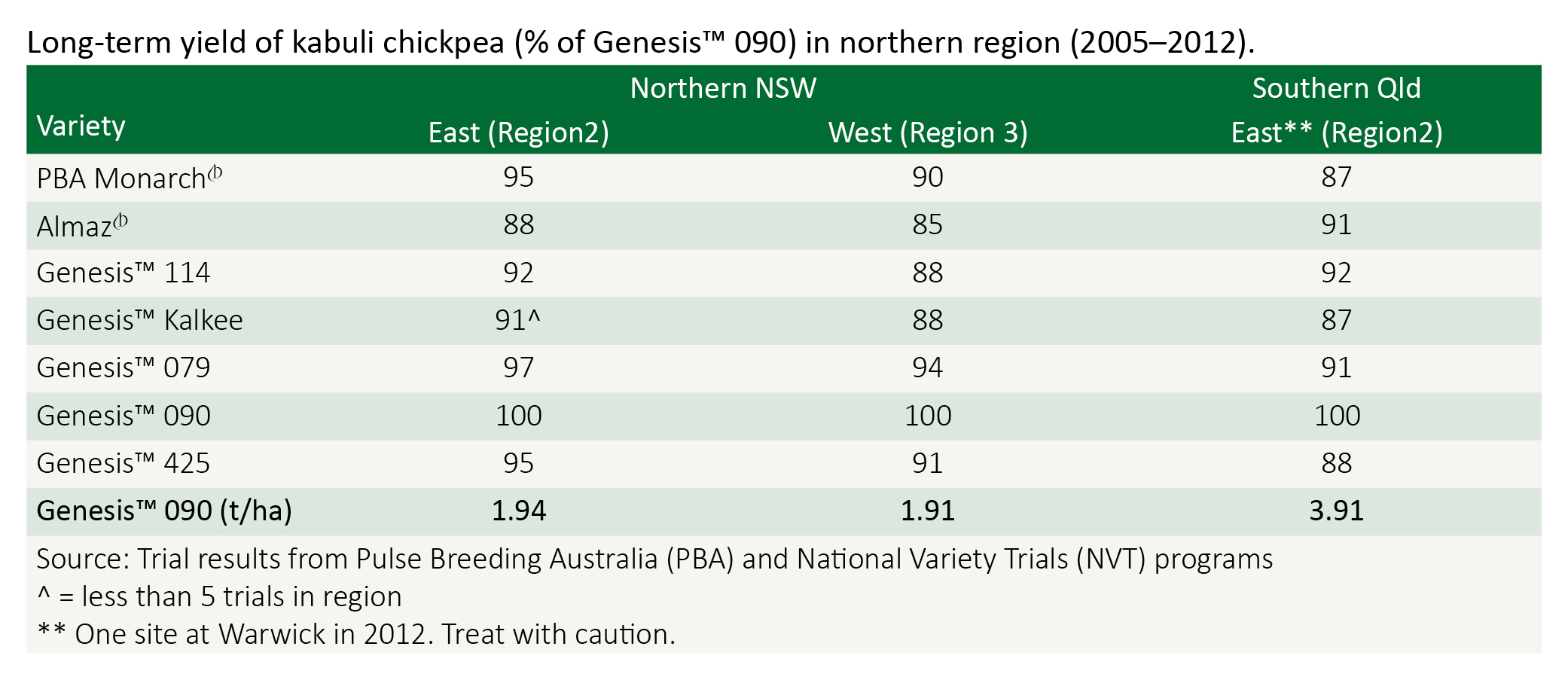
Almaz is a large seeded kabuli type, with seed size generally larger than Kaniva. It has superior ascochyta blight resistance than Kaniva, but inferior to that of Genesis 090. Seed is available through Seednet.
Area of Adaption: Zones 2 and 4. For more detailed information: Almaz Variety Management Package
Genesis 090 was the first kabuli type released in Australia with resistance to ascochyta blight. It is a small to medium seeded kabuli, having seed 7–8 mm in size, approximately 1 mm smaller than Almaz, Kaniva or Genesis 114. It is therefore is unlikely to attract the higher prices of these larger types. Genesis 090 has medium height with erect branches. It has shown to be a high yielding variety in both desi and kabuli trials, with and without ascochyta blight disease pressure. Under high ascochyta blight disease pressure Genesis 090 will require fungicide protection during podding to prevent seed blemish and potential yield loss. Seed is available through Australian Agricultural Crop Technologies.
Area of Adaption: Zones 2, 3, 4 and 5. For more detailed information: Genesis 090 Variety Management Package
Genesis 114 is a large seeded kabuli type with moderate resistance to ascochyta. It is likely to require strategic applications of fungicides during the year. Genesis 114 has larger seed than Kaniva (9–10 mm). Seed of Genesis 114 is available through Australian Agricultural Crop Technologies.
Area of Adaption: Zones 2 and 4. For more detailed information: Genesis 114 Variety Management Package
Genesis 425, like Genesis 090, is a small to medium sized kabuli type that is ascochyta blight resistant. Genesis 425 has phytophthora resistance similar to Howzat and superior to all other kabuli varieties and hence may provide a small to medium sized kabuli option for the northern areas of NSW. Genesis 425 is likely to require fungicide sprays for ascochyta blight only during podding to produce high quality, disease free seed. Seed of Genesis 425 is available through Australian Agricultural Crop Technologies.
Area of Adaption: Zones 2, 3, 4 and 5. For more detailed information: Genesis 425 Variety Management Package
Genesis Kalkee is a large seeded kabuli type with moderate resistance to ascochyta, similar to GenesisTM 114 from which it was selected. It has larger seed (9–10 mm) than GenesisTM 114 and Almaz. Seed of Kalkee is available through Australian Agricultural Crop Technologies.
Area of Adaption: Zones 2 and 4. For more detailed information: Genesis Kalkee Variety Management Package
Kaniva was the traditional kabuli chickpea standard in southern Australia, although its relative small seed size has made attaining a market premium for seeds over 9 mm in size difficult to achieve. Kaniva is susceptible to ascochyta blight and requires an intensive fungicide strategy to grow successfully. Almaz and Genesis 114 are now better large-seeded options. Kaniva is lower yielding than Genesis 090 in both the presence and absence of ascochyta blight.
Area of Adaption: Zones 2 and 4 but now redundant.
Kimberley Large was released by CLIMA in WA as a replacement for Macarena due to higher yields and larger seed size in the Ord River area. Kimberly Large, like Macarena, is susceptible to ascochyta blight and is only recommended for the Ord River and central Queensland regions.
Area of Adaption: Zones 1 and Ord.
Macarena is a Spanish variety grown almost exclusively in the Ord River area, where it produces very large seed that is highly sought after by buyers. Macarena is susceptible to ascochyta blight, extremely frost sensitive, very early maturing and has medium plant height.
Area of Adaption: Zones 1 and Ord.
Nafice is one of the largest seed sizes of the kabuli types, however a prevalence of green kernel affected its marketability, and so Almaz became the preferred variety. Nafice has superior ascochyta blight resistance than Kaniva, but inferior to that of Genesis 090. Nafice was originally available through Seednet.
Area of Adaption: Zones 2 and 4 but now redundant.
General agronomy
Paddock selection
- Chickpea crops should be separated from previous year’s crop by at least 500 m and up to 1 km in areas where old stubble is prone to movement i.e. down slope and on flood plains. This helps to reduce the spread of ascochyta blight, a foliar/stubble borne disease.
- Avoid paddocks with high weed burdens.
- Check herbicide use over the previous 12–24 months and seek ac=dice regarding any potential residue problems prior to sowing.
- Check soil tests to determine if the soil type is suitable for chickpea production, i.e. pH 5.2–8.0, loams to self-mulching clays, sufficient stored soil moisture, absence of herbicide residues and absence of constraints such as sodicity, salinity/chloride, high bulk density and potential for waterlogging.
- Avoid paddocks with uneven soil surface and/or obstacles such as sticks and stones that may impede harvest.
Aim to direct drill chickpeas into standing cereal stubble. Crops reliably yield 10% higher when established this way.
Uniformity of soil type, paddock topography, and surface condition of the paddock are all important criteria in assessing whether country is suitable for chickpea production. Harvest losses are much higher in rough or uneven paddocks, particularly in dry seasons when crop height is reduced. Sticks or rocks, eroded gullies or gilgais (‘melon’ or ‘crab’ holes) will prevent headers operating at low cutting height. This is particularly important when using headers with wide fronts. Small variations in paddock topography can lead to big variations in cutting height across a wide front and a subsequent increase in harvest losses.
Chickpeas can be very indeterminate in the northern region environment and moisture supply can significantly affect crop maturity. Changes in soil type and moisture holding capacity across a paddock can lead to uneven crop maturation, delayed harvesting and increased risk of weather damage or high harvest losses. Paddocks that have even soil types are relatively easier to manage, and are preferred for chickpeas.
Residual herbicide
In wheat-chickpea rotations avoid the use of fallow and in-crop residual herbicides such as Broadstrike®, Eclipse®, Flame® Grazon®DS, Lontrel® and metsulfuron (Ally®, Associate®, Lynx®) Harmony®M, particularly during the summer fallow or weed control period (after November).
The use of long-term residual SU herbicides such as Monza®, chlorsulfuron (Glean®, Lusta®) and Logran® in wheat should be avoided when re-cropping to chickpeas.
For more detailed information: Herbicide residues in pulses
Stubble retention
Chickpeas fit well into stubble retention systems with no tillage, and serve their wider role in crop rotations and farming systems. Retention of adequate plant residues on the surface is important to protect the soil from erosion both during growth and after harvest. This will not affect chickpea germination and growth, and can improve establishment on hard-setting, surface-crusting soils. Sowing into cereal stubble reduces soil moisture losses from evaporation.
For more detailed information: Wide rows and stubble retention
Paddock preparation
Chickpea seed is best sown into friable soil, with direct drilling often possible following a cereal crop. Good depth and adequate seed-to-soil contact is required and the large seed size of chickpeas assists in this regard.
There are few problems when sowing desi and most kabuli chickpeas with conventional seeding equipment, but occasionally cracking of seed may occur with the larger seeded kabuli types.
Inoculation
Pulses have the ability to ‘fix’ their own nitrogen from the air via nodules on their roots if specific nitrogen fixing bacteria (rhizobia) are available. The strain of rhizobia used for chickpeas is highly specific (Group N – CC1192) and inoculation is essential for effective nodulation. The Group N bacteria are regarded as an ‘aggressive nodulator’. This effectively means that nodulation will be successful in meeting the crop’s nitrogen requirements provided the inoculants are handled and stored in a manner that will ensure bacterial survival and that growers adopt effective inoculation practises on-farm.
Inoculated seed must be planted into moisture within 12 hours of treatment. The sooner the better, as fungicide seed dressings can effect survival of the bacteria.
For more detailed information: Inoculating pulse crops
Sowing
High quality seed is essential to ensure the best start for your crop. Grower retained seed may be of poor quality with reduced germination and vigour, as well as potentially being infected with seed-borne pathogens.
- All seed should be tested for quality including germination and vigour.
- If grower retained seed is of low quality then consider purchasing registered or certified seed from a commercial supplier and always ask for a copy of the germination report.
- Regardless of the source, treat seed with a thiram-based fungicide.
- Careful attention should be paid to the harvest, storage and handling of grower retained seed intended for sowing.
- Calculate seeding rates in accordance with seed quality (germination, vigour and seed size).
For more detailed information: Chickpea: High Quality Seed
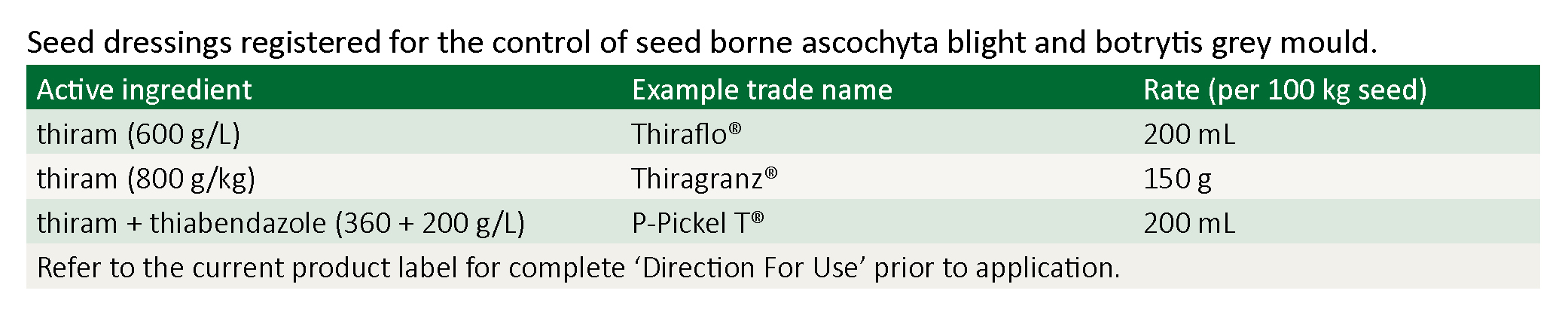
Seed treatment
A fungicidal seed dressing to suppress early development of ascochyta blight is also essential. Use thiram or thiabendazole and thiram combined, which is also effective against botrytis grey mould.
Seed testing
High quality seed is vital. Check seed labels for germination percentage and purity and ask for the germination certificate. The results of a germination test must be supplied with all seed for sale. Take the additional precaution of having the seed tested for both ascochyta and botrytis grey mould. Harvesting on time minimises the development of disease on seed.
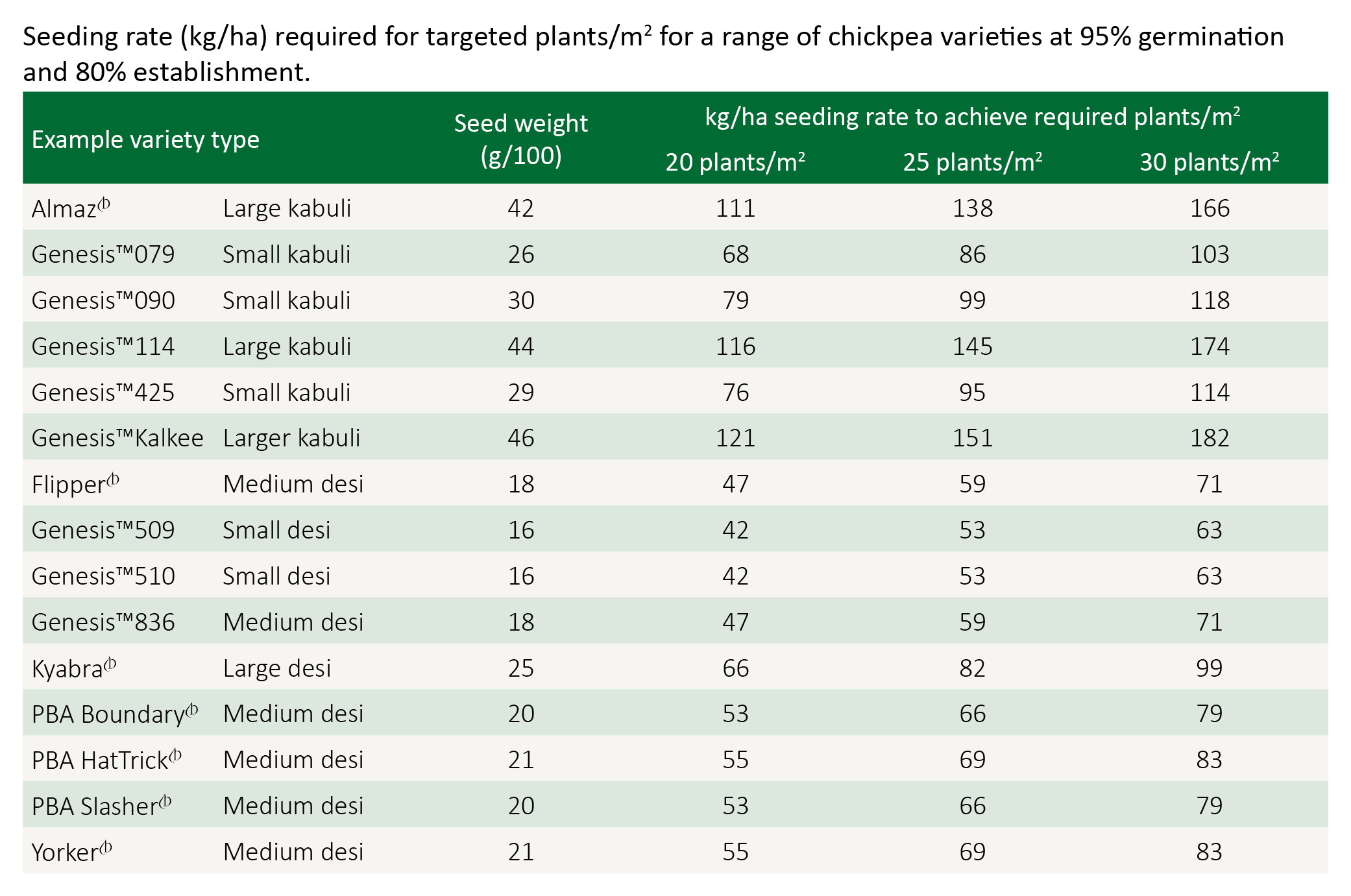
Sowing rate
While yields are relatively stable within the range of 20–40 plants/m2, populations of 30 plants/m2 will optimise yields in the northern region. Research has shown that slightly higher populations are required in relatively colder production areas in northern NSW.
Higher populations are justified for late plantings, while lower populations of around 20 plants/m2 are often recommended for crops grown on wide row spacings (1 metre). High populations planted on wide rows often result in thin main stems and a higher risk of lodging.
Seeding rate for the target plant density can be calculated using germination percentage, 100 seed weight and establishment percentage.
Sowing depth
Sow chickpeas 5–7 cm deep into good moisture. The seedlings are robust, provided high quality seed is used. The agronomic advantages of sowing at 5–7 cm include:
- reduced risk of damage from pre-emergent residual herbicides such as simazine, Balance etc.
- improved early formation of lateral roots in the top soil
- enhanced inoculum survival in moist soil
- a significant proportion of ascochyta infected seed is eliminated due to high mortality of diseased seed.
Avoid sowing deeper than 7 cm on soils prone to surface sealing and crusting.
Press-wheels can improve establishment, although heavy pressures should be avoided. V shaped press-wheels will leave a furrow down the planting line that can lead to a concentration of residual herbicides in the furrow after rainfall and subsequent crop damage.
Deep planting
Many growers have been deep planting chickpeas for some time. Excellent plant emergence has been achieved from up to 15 cm deep, and planting depth can be varied from 5–20 cm according to seasonal conditions. Deep planting is not only an extremely valuable tool under drought conditions, but can also offer major advantages in most years including:
- enabling planting at the optimum time
- freeing up valuable time for planting wheat when suitable planting rains fall
- avoiding residual herbicide damage
- better development of lateral roots
- improved nodulation.
For more detailed information: Chickpeas: Deep planting strategies
Row spacing
Chickpeas are successfully grown using a wide range of planting equipment and row spacings ranging from 18 cm to 1 metre. Stubble retention, preferably standing stubble, is essential with wide rows.
The recent trend is toward an increasingly higher proportion of the northern region crop being grown in either:
- wide rows of 0.5 - 1.0 metres.
- controlled traffic layouts with a modified ‘broadacre’ configuration.
Wide rows (50–100 cm) offer:
- Greater ability to plant into heavy stubble cover. Zero tillage systems have shown a consistent 10–15% yield advantage over cultivated systems.
- Precision planters often provide more accurate seed placement, resulting in better establishment and more even plant stands. This often results in more even crop maturity.
- Improved harvestability due to plants being more erect, with a higher pod set as a result of ‘within row’ plant competition. This is particularly important in low yielding situations.
- In low yield situations, crops planted on wide rows often ‘feed in’ better over the knife section of the header due to the concentration of growth within the row.
- Reduced input costs through band-spraying of insecticides and defoliants.
- Relatively cheaper weed control using glyphosate through shielded spraying equipment.
- Easier access and ‘marking’ for ground spraying pesticides and desiccants in permanent controlled traffic (CT) lanes.
- Better yields under severe moisture stress conditions attributed to the combination of wide-rows and heavy stubble cover than narrow rows configurations.
- Easier access to the crop when checking for pests such as helicoverpa.
- Improved air circulation in the crop, which lowers humidity levels and can reduce the severity of foliar fungal diseases.
- Allows interow cultivation and ‘directed’ herbicide sprays e.g. Broadstrike®.
Narrow rows (15–40 cm) offer:
- Potential yield advantage at yields levels above 1.5 t/ha. Any yield advantage is often negated however, by the inability to maintain a zero-till system when planting on narrow row spacings.
- Relatively fewer lodging problems in high yield situations.
- Suits conventional wheat planting equipment.
For more detailed information: Wide rows and stubble retention
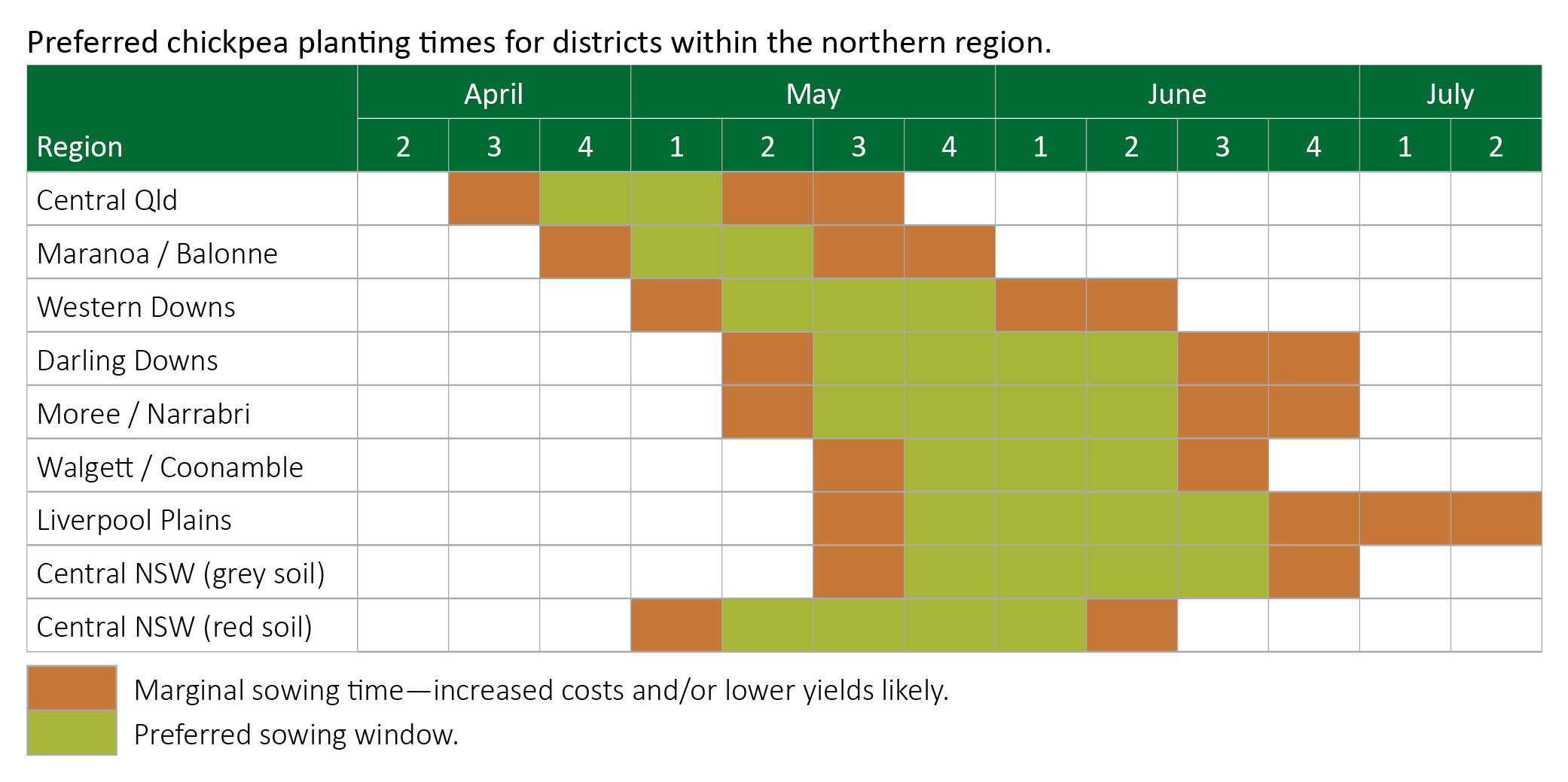
Sowing time
Chickpea shows a marked response to time of sowing. Crops sown ‘on time’ have an excellent chance of producing very high yields. However, crops sown earlier or later than recommended often suffer reduced yields.
Water use efficiency is commonly in the range of 8–12 kg grain/ha/mm for sowings made during the preferred sowing window. This drops away to 4–6 kg/ha/mm for very late or very early sowings.
Chickpea seedlings are tolerant of frost. Desi chickpea seed can germinate in soil as cold as 5°C, but seedling vigour is greater if soil temperatures are at least 7°C. Kabuli chickpea seed is more sensitive to cold soils and should not be seeded into excessively wet soil or into soil with temperatures below 12°C at the placement depth.
Seed treatment is very effective against seed rot, permitting early seeding of kabuli types to help offset the later maturity of currently available kabuli chickpea varieties. Because the large-seeded kabuli varieties mature later than the desi varieties, they may need to be sown earlier than desi in some districts.
If the seed is treated, it should be planted immediately after inoculation, as seed treatments can be toxic to the inoculant. The longer the inoculant is in contact with the seed treatment, the less effective it will be.
Nutrition
Chickpeas are adapted to alkaline soils with high levels of unavailable P, and have evolved methods of extracting P, and some other nutrients, from the soil that would be inaccessible to many other pulse and cereal crops.
This ability is largely due to a combination of two factors: organic acids secreted from the root system and arbuscular mycorrhizalfungi (AMF) colonising the chickpea root system increasing uptake of P and Zn. More P may be required in low AMF situations (e.g. after a long fallow).
Chickpea is considered highly dependent on AMF to reach yield potential so yield reduction of 60–80% can occur in low AMF situations. For more detailed information: VAM and long fallow disorder (Qld DAF)
Inoculated seed and acidic fertilisers should not be sown down the same tube. The acidity of some fertilisers will kill a high proportion of the rhizobia and render inoculation ineffective. Neutralised (e.g. Super lime) and alkaline fertilisers (e.g. DAP, Starter NP, lime) can be safely used.
Phosphorus
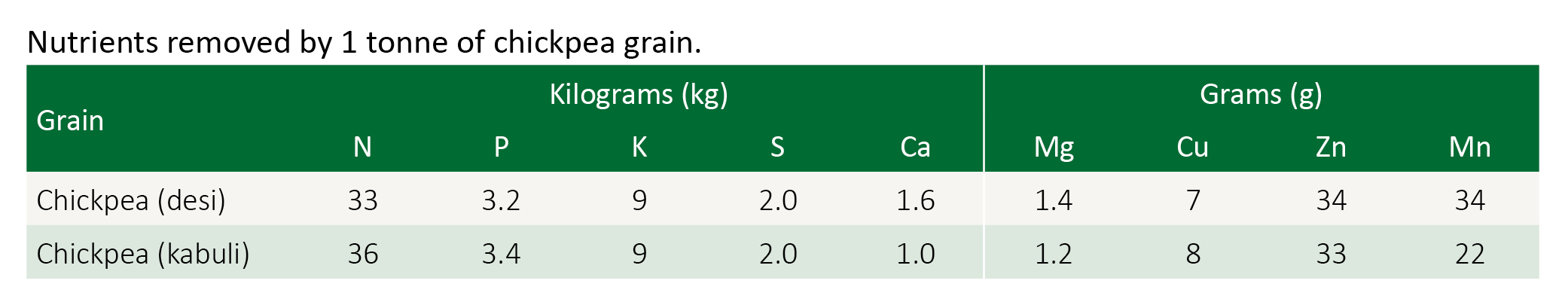
Soil phosphorus levels influence the rate of nodule growth. The higher the phosphorus level the greater the nodule growth. A 2 t/ha chickpea crop will on average remove approximately 6.5 kg/ha of phosphorus. This then is the minimum amount of phosphorus that needs to be replaced. Higher quantities may be needed to build up soil fertility or overcome soil fixation of phosphorus.
Chickpeas are not as responsive to phosphorus fertiliser as some of the other pulses. In order to match the nutrient requirement of a crop yielding 1.5-3.5 t/ha, a guide for alkaline soils with a good fertiliser history is 7-16 kg/ha of phosphorus (P). This is equivalent to 80-186 kg/ha of single super or 40-95 kg/ha of double super.
Sulphur
Sulphur (S) is needed at higher rates for chickpea. Use "grain legume" fertilisers. If the paddock has a history of single super then S may be adequate, particularly on clay soils. Prolonged use of double or triple super could lead to an S deficiency, especially on lighter soils.
Nitrogen
Nitrogen fertilisers in small amounts (5–15 kg N/ha) are not harmful to nodulation and can promote early root growth to establish stronger plants. Fertiliser compounds such as MAP and DAP are suitable for chickpea production. Excessive amounts of nitrogen however, will restrict nodulation and reduce nitrogen fixation.
‘Starter N’ may be beneficial, but is not essential.
Zinc and other micro-nutrients
Chickpea is considered to have a relatively high demand for zinc, but also possess highly efficient mechanisms for extracting Zn from the soil. Zinc seed treatments may be a cost-effective option in situations where soil P levels are adequate but zinc levels are likely to be deficient.
Chickpeas are prone to zinc (Zn) deficiency. Low or marginal zinc levels are widespread in many cropping districts. Zinc, and to a lesser extent iron, deficiency is prevalent on calcareous soils, particularly dark brown clay soils with high pH.
Zinc applications lasts about 2 years on calcareous clays and 6–7 years on loamy soils. Zinc is not mobile in the soil and an even distribution is important. Zinc can be applied by spray to the soil, in furrow, coated on granular fertiliser or as a foliar spray.
Molybdenum and cobalt are required for effective nodulation and should be applied as needed.
Boron
Chickpeas are considered sensitive to boron toxicity. Symptoms show as a yellowing or dying of the tips and margins of the leaves, with the older leaves being more severely affected than younger leaves.
Irrigation
Full or supplementary irrigation of chickpea is common in districts where chickpea is grown in rotation with other irrigated crops, such as cotton. Management requirements for irrigated chickpea are the same as for dryland, but their sensitivity to waterlogging, for even a short time, can result in severe losses, particularly if the crop is also under stress from herbicides or disease.
Using sprinkler irrigation equipment reduces the risk of waterlogging, even during flowering and pod-fill, however there may be a higher risk of foliar disease, e.g. botrytis grey mould and ascochyta blight, due to the increased irrigation frequency and leaf wetness.
Factors to consider when planning for irrigated chickpea production include:
- Avoid heavy clay or dense soil types (bulk density >1.5) that do not drain freely and are subject to waterlogging.
- Select fields with an effective irrigation layout, such as beds or hills, and relatively good grades.
- A border-check layout that is steeper than 1:800 grade is suitable provided there are short runs on free draining soils that can be irrigated quickly and do not remain saturated.
- Rolling may be required to flatten the ridges left by press wheel furrows or to flatten clods.
- Irrigation can be used in activating and incorporating a number of pre-emergent herbicides.
- Pre-irrigate to fill the moisture profile prior to planting chickpea crops, unless there has already been sufficient rainfall. Watering up is most effective in bed, row and sprinkler systems, but is not recommended for border-check layout unless soil moisture is insufficient to achieve a uniform germination.
- As a general rule, irrigation of the emerged crop should start early when there is a deficit of between 30–40 mm and around 60–70% field capacity. Schedule irrigation using soil moisture indicators rather than the crop growth stage.
- Time irrigation application to prevent moisture stress during flowering and podding and to reduce the impact of high temperatures on yield, quality and grain size. This is particularly important with large kabuli types. Chickpea is very sensitive to waterlogging during flowering and podding so great care is required to provide adequate soil moisture without causing waterlogging.
- In furrow irrigation systems, water every second row to avoid waterlogging. Doubling up the number of siphons can increase water flow and reduce irrigation time.
- Aim to have watering completed in less than eight hours, and have good tail water drainage to avoid any waterlogging in the crop area.
- Avoid irrigating if rain is forecast for the near future.
- In border-check layouts and paddocks with heavy soil types or long runs: if in doubt, do not water.
Irrigation management strategy for chickpea
- Pre-irrigate to fill the moisture profile prior to planting chickpea crops, unless there has already been sufficient rainfall. Watering up is most effective in bed, row and sprinkler systems, but is not recommended for border-check layout unless soil moisture is insufficient to achieve a uniform germination.
- As a general rule, irrigation of the emerged crop should start early when there is a deficit of between 30–40 mm and around 60–70% field capacity. Schedule irrigation using soil moisture indicators rather than the crop growth stage.
- Time irrigation application to prevent moisture stress during flowering and podding and to reduce the impact of high temperatures on yield, quality and grain size. This is particularly important with large kabuli types. Chickpea is very sensitive to waterlogging during flowering and podding so great care is required to provide adequate soil moisture without causing waterlogging.
- In furrow irrigation systems, water every second row to avoid waterlogging. Doubling up the number of siphons can increase water flow and reduce irrigation time.
- Aim to have watering completed in less than eight hours, and have good tail water drainage to avoid any waterlogging in the crop area.
- Avoid irrigating if rain is forecast for the near future.
- In border-check layouts and paddocks with heavy soil types or long runs: if in doubt, do not water.
Yield expectation
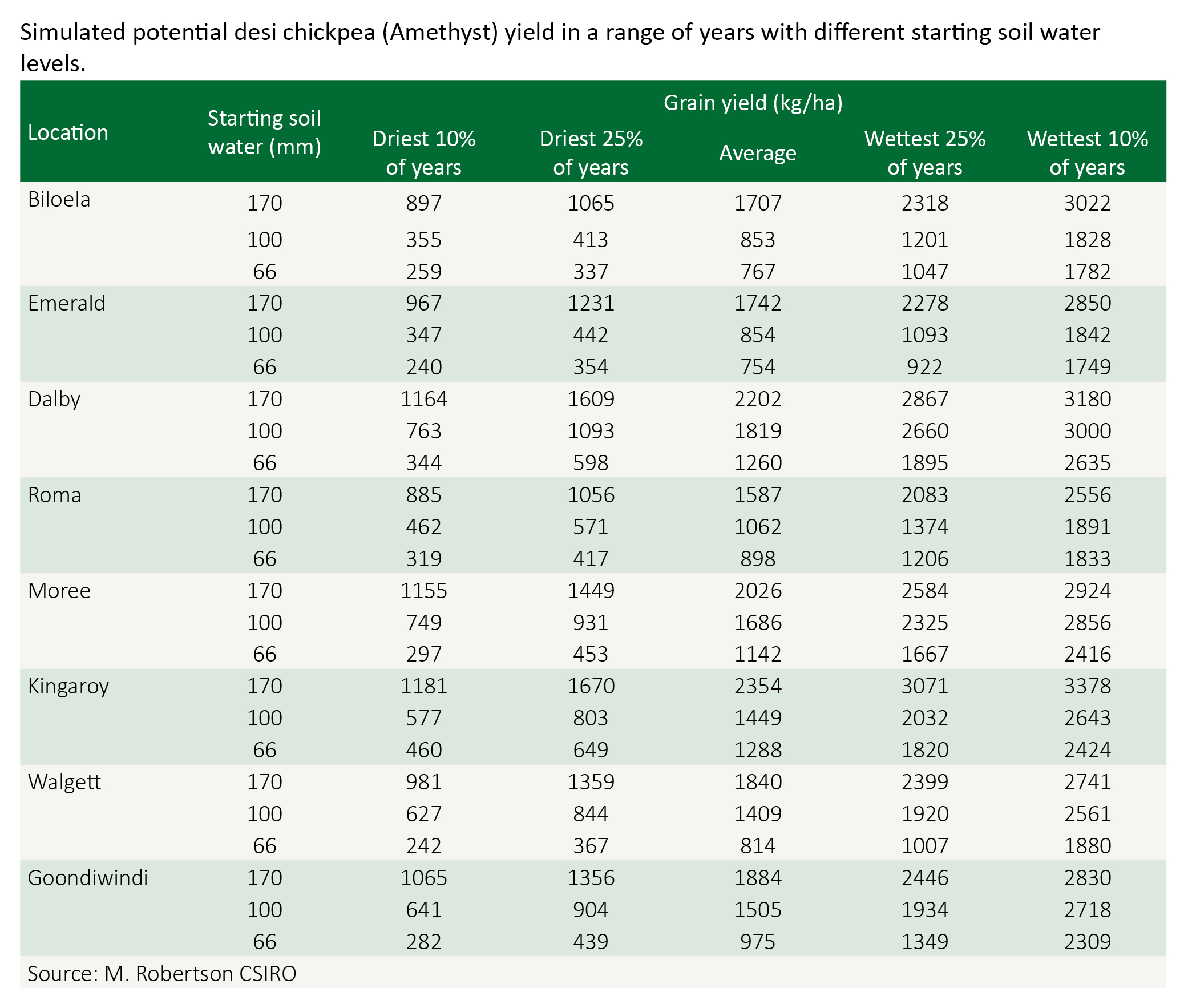
Starting soil water can have a strong influence on the yield expectation of chickpea as well as the riskiness of production. The following table gives the average and range of yields in good/poor years.
Note: The figures are derived from the simulation model APSIM, which has been tested in the northern region for over 10 years. Figures in the table are APSIM-simulated chickpea yields for three conditions of starting plant available water at 8 locations in the northern grains region. Simulations were conducted with 100 years of daily historical climate data at each location. The simulation setup involved cv. Amethyst, sown in late May at 20 plants/m2. The figures shown are a conservative estimate.
Weed management
Chickpea crops are poor competitors with weeds because of their slow emergence and growth during winter. Effective weed control is essential to prevent yield loss and to avoid the build-up of troublesome weeds in the rotation.
For best results, sow chickpea into paddocks with low broadleaf weed populations. Make the most of opportunities to reduce broadleaf weeds in the preceding crop when weed control is likely to be more effective, cheaper and cause less damage to that crop. Delaying chickpea sowing until after a germination of broadleaf weeds also assists in areas or seasons where this is possible.
The over-use of particular groups of herbicides through the rotation can lead to herbicide resistance, which has occurred in grass weeds and now some broadleaf weeds. To avoid herbicide resistance, weed management through the rotation should aim to minimise the need for herbicides, to avoid the overuse of any one group of herbicides and to use the least selective herbicide. Effective grass control in the chickpea crop has the benefit of reducing the need for selective grass herbicides in the following cereal year.
Balance® (isoxaflutole) is a Group H herbicide and its use is specific to chickpea for broadleaf weed control in broadacre cropping situations. It provides a weed control option unique to chickpea and enables rotation of herbicide groups across the cropping sequence.
Chickpeas can be grown in wider rows in a stubble system that allows inter-row herbicide application with shielded sprayers. For more detailed information: Wide rows and stubble management
Problem weeds or situations that require special attention include:
- Group A (‘dims’ and ‘fops’) resistant wild oats (and other grass species).
- Late germinations of weeds (e.g. ryegrass, brome grass) that would normally be prevented from setting seed in other pulses through croptopping.
- Snail medic, which can escape Balance®.
- Hoary cress, soursob, tares and volunteer pulses.
Avoiding herbicide damage
Some herbicides can severely damage chickpea crops through residues in soil, contaminants in spray equipment, spray drift onto the crop or by incorrect use of the herbicide.
The importance of cleaning and decontaminating spray equipment before the application of herbicides cannot be over-stressed. Traces of sulfonylurea herbicides (such as chlorsulfuron, metsulfuron or triasulfuron) in spray equipment can cause severe damage to chickpea and other legumes when activated by grass control herbicides.
Pulse crops can be severely damaged by some hormone herbicide sprays, such as 2,4-D ester, drifting into the crop. This can happen when these sprays are applied nearby in very windy or still conditions, especially where there is an inversion layer of air on a cool morning.
Taking some general precautions can help to reduce the likelihood of crop damage with residual herbicide use at planting:
- Do not apply residual herbicides if rain is imminent.
- Maintain at least 7.5–10 cm soil coverage.
- Avoid leaving a furrow or depression above the seed that could allow water (and chemical) to concentrate around the seed or seedling.
- Avoid leaving an exposed, open slot over the seed with disc-openers and avoid a cloddy, rough tilth with tined-openers.
For descriptions and pictures of herbicide injury refer to ‘Field crop herbicide injury: The Ute Guide’ and ‘Chickpea Disorders: The Ute Guide’ both are available from GRDC Groundcover direct.
Herbicide options
Chickpeas are late maturing (compared to other pulses), hence croptopping to prevent ryegrass and other weed seed set is not possible, even in the earliest of maturing varieties (e.g. Genesis 079). Chickpeas are relatively slow to emerge with slow early growth during the colder winter months. As a consequence, they are poor competitors with weeds. Even moderate weed infestations can cause large yield losses and harvest problems.
Trials in northern New South Wales and central Queensland have shown that populations of five to ten turnip or ten wild oats per square metre can cut yields by as much as 40–60% (D White 2000 and J Whish 1998). Because of the slow growth and open canopy in chickpeas, narrow or wide row spacing (30 v 70 cm) made little difference to the chickpea plant’s ability to compete with weeds.
The weed control strategy for growing a successful chickpea crop depends on substantially reducing the viable weed seed bank in the soil before the crop emerges. Control the majority of weeds before seeding, either by cultivation or with knockdown herbicides such as glyphosate or Spray•Seed®.
A technique used with varying success by growers has been to sow chickpea and then use a knockdown herbicide tank mixed with a pre-emergent herbicide to control germinating weeds before the crop has time to emerge. Chickpea crops may take up to 21 days to emerge under cool, drying soil conditions but under favourable warm, moist soil conditions plants may emerge after 7 days. Growers considering this option should sow deeper (10–15 cm) and carefully check their paddocks for the emergence of the chickpea immediately before spraying. Done well, this can be an effective weed control option.
The absence of cost-effective and safe post-emergent herbicides effectively limits broadleaf weed control options in chickpeas to a small number of pre-emergent herbicides. Most of these chemicals are very dependent on rainfall soon after application, and as a consequence often result in inconsistent or partial weed control under drier conditions.
The pre-emergent herbicides will not adequately control large weed populations by themselves, and so they need to be used in conjunction with paddock selection and pre-seeding weed control. Incorporation by sowing (IBS) is generally considered safer on the crop than Post-sowing Pre-emergence with most herbicides used in modern no till sowing systems. Most of these products work best if thoroughly mixed with soil either mechanically or by irrigation or rainfall. The aim of incorporation is to produce an even band of herbicide to intercept germinating weed seeds.
Simazine is the most widely used herbicide for broadleaf weed control, and can provide relatively cheap control of cruciferous weeds (e.g. sowthistle). Efficacy is very dependent on receiving rainfall (20–30 mm) within 2–3 weeks of application, and consequently weed control is often disappointing under drier conditions.
Balance® (isoxaflutole) is a systemic herbicide belonging to the relatively new class of isoxazole herbicides (Group H). Balance provides more consistent and reliable control of susceptible weeds for longer and across a broader range of seasonal conditions.
Terbyne is the newest triazine herbicide to be introduced in Australia and is registered for pre-emergent weed control in chickpea, lupin, field pea, faba bean, lentil and triazine tolerant canola. Terbyne is recommended for pre-emergent use (pre or post sowing).
Terbyne controls a wide range of broadleaf weeds, with some suppression of grasses, particularly if there is good soil moisture. Sufficient rainfall (20–30 mm) to wet the soil through the weed root zone is necessary within 2–3 weeks of application.
Spinnaker® (700 g/kg imazethapyr) is NOT REGISTERED FOR USE IN CHICKPEA CROPS IN NSW OR QUEENSLAND.
In chickpea crops sown on wide rows, there is increasing adoption of ‘directed sprays’ of Broadstrike, either alone or in tank-mixes with simazine. This largely avoids the problem of crop damage and improves weed control through the ability to safely add wetters or mineral oils to the spray mix.
Directed sprayers are most common in or around the cotton growing areas, as they enable relatively cheap grass and broadleaf weed control using glyphosate in-crop. While chickpeas do have a degree of tolerance to glyphosate during the vegetative stage, caution is still required as the lower branches arising from the main stem contribute a large proportion of the total chickpea yield. Upright varieties such as Amethyst and Jimbour are more suited to this technique than the more prostrate types and small chickpea plants are more susceptible to damage than older plants.
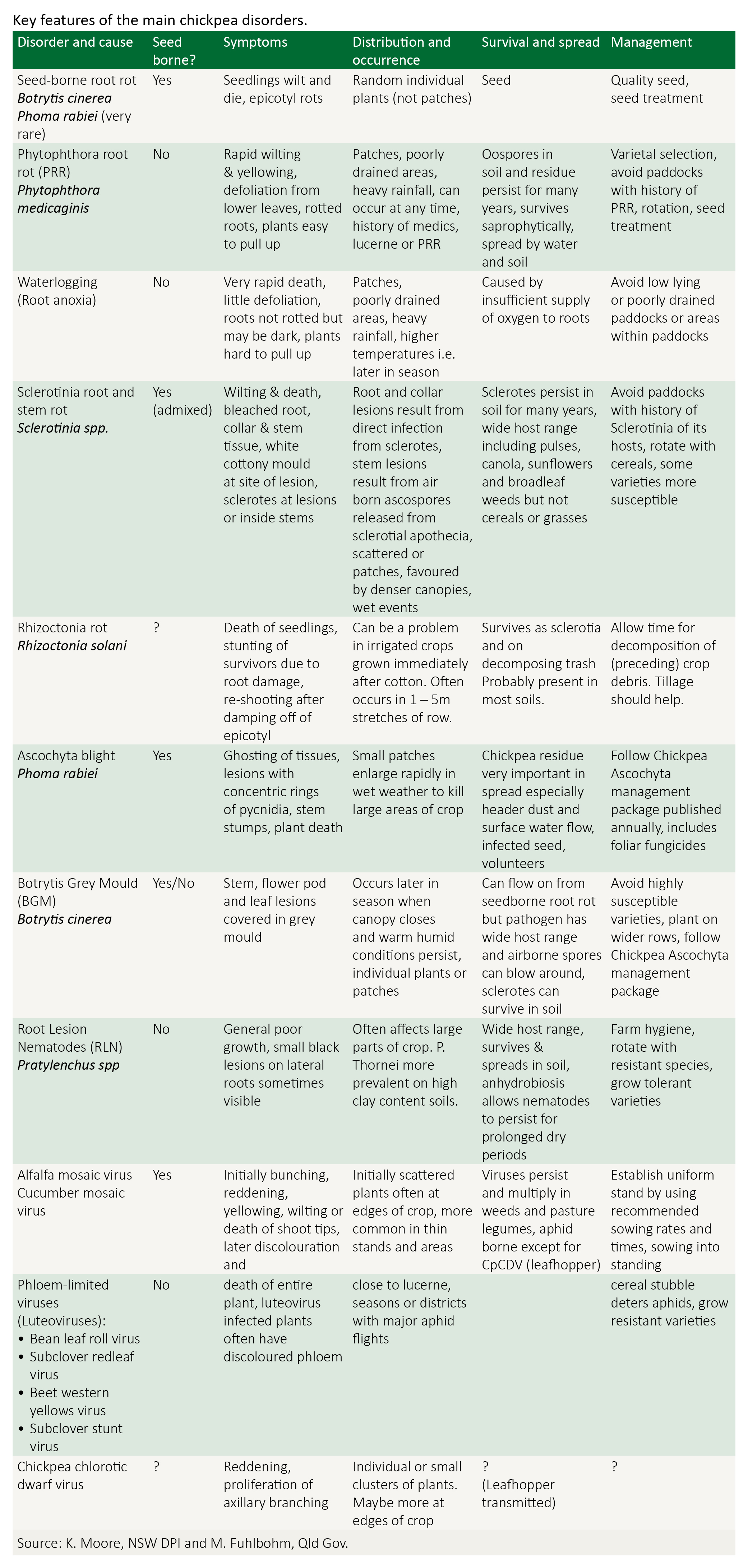
Disease management
Several foliar fungal diseases, some seedling root diseases, viruses and root lesion nematode can affect chickpea. Disease management of chickpea should primarily focus on ascochyta blight. Phytophthora root rot is a most damaging disease in northern NSW and Queensland.
Be familiar with and follow the integrated disease management strategies recommended for chickpea.
For specific information related to identifying and managing each crop, refer to the following resources:
- Ascochyta blight
- Botrytis grey mould
- Phytophthora root rot
- Sclerotinia
- Root lesion nematodes
- Viruses
Control of fungal diseases
Sprays will control fungal disease, but when and how often to spray will depend on the varietal resistance, amount of infection, the impending weather conditions and the potential yield of the pulse crop.
Fungal disease control is geared around protection rather than curing. The first fungicide spray must be applied as early as necessary to minimise the spread of the disease. Additional sprays are required if the weather conditions favour the disease.
A fungicide spray at the commencement of flowering protects early pod set. Additional protection may be needed in longer growing seasons until the end of flowering. Fungicides remain effective for approximately 2–3 weeks. Keep in mind that all new growth after spraying is unprotected. In periods of rapid growth and intense rain (50 mm over several days) the protection period will reduce to around 10 days.
Timing of fungicide sprays is critical. As ascochyta and botrytis can spread rapidly, DO NOT DELAY spraying. A spray in advance of a rainy period is most desirable. Despite some fungicide washing off, the disease will be controlled. Delaying until after a rainy period will decrease the effectiveness of the fungicide as the disease has started to spread.
The need for repeated fungicide sprays depends on the amount of unprotected growth, the amount of rainfall since spraying and the likelihood of a further extended rainy period. Unprotected crops can lose over 50% in yield. In severe cases the crop may drop all its leaves.


Problem solver
Pest management
Establishment pests
Chickpea exude malic acid from their leaves and stems making them taste quite bitter and unpalatable to most seedling pests. Chickpea is therefore quite tolerant to attack from some pests such as redlegged earthmite, lucerne flea, cutworm and pasture looper. Damage to chickpea crops can still occur due to the lack of more palatable alternatives. Chickpea crops should be checked during and after emergence for these and other invertebrate pests so that early action can be taken if plants are being chewed and crop density is being reduced.
Flowering and podding pests
Native budworm (Helicoverpa) larvae is the main insect pest of chickpeas. It is important to be able to identify the different larval instars (very small, small, medium, large) of helicoverpa.
There are two species of helicoverpa, H. armigera and H. punctigera. H. armigera is resistant to some insecticide groups, whilst H. punctigera is susceptible to all products. While it is not always possible to do so, identifying which species is present, or knowing which is predominate in your area, may help you avoid products that may not provide adequate control.
If the level of H. punctigera is high, any registered product will control the larvae. If H. armigera is the dominant species, spray failures with carbamates or pyrethroids may occur due to resistance. The biopesticides Helicoverpa nucleopolyhedrovirus (NPV) and Bacillus thuringiensis (Bt) currently have no known resistance problems.
Helicoverpa do most damage during pod set through to maturity, and can reduce both grain yield and quality.
Regular monitoring of the crop for the presence of insects pests and/or damage is necessary in order to make timely decisions on control, especially when it is important to be targeting small and possibly insecticide resistant larvae.
Sampling with a beat sheet is best practice for monitoring helicoverpa in chickpeas.
Experienced agronomists suggest that helicoverpa numbers alone do not always give an accurate assessment of damage being caused. In some situations (e.g. if the crop is severely moisture stressed), monitoring crop damage (e.g. number of damaged vs undamaged pods) will assist in assessing the accumulating yield loss.
Check crops regularly looking for helicoverpa larvae, usually once per week prior to pod set and 2–3 times a week from pod set onwards. Activity of adult moths in the crop, and the presence of eggs may be indicative of future larval activity.
Close monitoring can pay-off. In many cases the larval infestation may not progress past the ‘smalls’ stage and therefore control is unwarranted. Regular close checking, and reference to records from successive checks, will enable you to determine larval survival.
Aim for one well-timed spray. Chickpea can tolerate moderate to high numbers of helicoverpa larvae (10–20 larvae/m2) through the late vegetative and early flowering growth stages. Most yield loss will be sustained from damage caused during pod fill, and this is the most critical stage for crop protection.
Larval infestations are likely to be of mixed ages by the time the crop is well into podding. Products like StewardTM and Larvin® will adequately control a wide range of larval sizes. These products also offer approximately 10–14 days of residual protection when applied to plants that are not actively growing.
Biopesticides (NPV and Bt) must target smaller larvae (preferably less than 7 mm in length). Therefore, in situations with high larval densities across a range of size classes, biopesticides are not the preferred choice. For more detailed information: Using NPV in field crops (QDAF)
Keep pod damage in perspective. While larvae and their damage may appear very evident in a crop, counts of damaged and undamaged pods will give an estimate of actual yield loss accumulating (1 damaged pod per m2 = 1.7 kg/ha yield loss). In most cases relative pod damage is far less than initial visual inspections suggest, so careful monitoring of damage, in relation to total pod load, is recommended. Once pods are damaged, however the yield is already lost so assessing pod damage is not useful in making control decisions to prevent yield loss.
Be aware of withholding periods. Both Steward and Larvin have long withholding periods (21 days), as do some other products. Be aware that late sprays of these products could therefore delay the harvest date.
For more detailed information: Managing native budworm in pulses
Aphids and viruses
The malic acid in chickpeas does mean that there is little aphid colonisation of chickpea plants. However, bluegreen aphids and cowpea aphids both transmit cucumber mosaic virus (CMV), a non-persistent virus, while visiting chickpea crops. Cowpea aphids also transmit beet western yellows virus (BWYV), a persistent virus, while visiting chickpea crops. Hence management of chickpea crops and protection against aphid from invasion from surrounding areas is important.
For more detailed information: Virus management in pulses
Locusts and grasshoppers
Locusts and grasshoppers will cause damage to chickpeas in the same way that they will cause damage to any green material when in plague numbers. Chickpeas may however be less vulnerable in the seedling stages compared to lupins and lentils. Sheer weight of numbers can however lead to significant damage.
Most locust plagues originate in south west Queensland and adjacent areas of South Australia, New South Wales and the Northern Territory. Locust populations develop following rainfall in this area.
With suitable conditions, autumn swarms may migrate 200–500 km into pastoral and adjacent agricultural areas. On arrival they lay eggs, which hatch to produce the spring outbreak.
For more detailed information: Locust damage in pulses
Desiccation, harvest and storage
Harvest delays in chickpeas cost growers and the pulse industry a lot of money. In any production area it is not unusual to see up to a 4–6 week spread in the harvesting of chickpea crops planted on the same sowing rain. Often, many of the late harvested crops are down around 8% moisture content, whereas the maximum moisture content for receival is 14% and the preference is for 12%.
Chickpea harvest clashes with wheat harvest on many farms however growers who prioritise chickpea harvest operations are rewarded with premiums for the high quality grain produced. The premiums available for early harvest chickpea are often considerably greater than those for PH/AH wheat.
Some growers have a perception that chickpea crops ‘weather’ reasonably well compared to other crops but this is not really the case. Chickpea grain quality does deteriorate if harvest is delayed.
Uneven ripening in chickpea crops can be a problem, especially on heavy clay soils. Growers can plan ahead for timely desiccation and harvest to maximise yield and quality potential. Desiccation also aids in harvestability of the crop.
Grain quality deteriorates the longer mature chickpeas are exposed to weathering in the field. The effects of delayed harvest include:
- cracking of the seed coat
- increased mechanical damage during harvest and handling
- increased costs associated with grading out defective grain and lost potential income
- increased risk of disease and mould infection that can lead to consignment rejection.
Early harvested chickpea seed is much more resilient to breakage during harvesting and subsequent handling, even at low moisture contents.
The chickpea seed coat is very prone to cracking if it has been exposed to wetting and drying events due to rain or heavy dew. Expansion of the seed as it absorbs moisture, and then contraction as it dries, weakens the seed coat, rendering it much more susceptible to mechanical damage during harvest and handling operations. Levels of cracked and damaged grain can be as high as 50% in extreme cases of field weathering and prolonged rainfall.
Chickpeas that do not meet the Export Receival Standard of 6% max Defective Chickpea will need to be graded.
Desi chickpeas are ultimately processed into dahl by removing the seed coat (hull) and splitting the cotyledons. This process uses abrasive type mills to gradually abrade the seed coat from the cotyledons, and is reliant on the seed coat being firmly attached to the cotyledons. Dahl is often further processed into ground products and flour.
Cracking and weakening of the seed coat prior to processing substantially reduces the recovery percentage (%) of dahl, as well as reducing the quality of the final product.
Field weathered chickpeas after rain are also more difficult to thresh out at harvest, and often contain much higher levels of admixture such as unthreshed pods and pod material, which adds to storage risks and cleaning costs.
Chickpea seeds also discolour and darken when exposed to field weathering. Darkening of the seed coat is caused by oxidation of polyphenol compounds when the seeds are exposed to rainfall, cool-mild temperatures and or high humidity.
While there is usually no direct penalty or discount for a moderate degree of seed coat darkening, it does have a significant impact on the marketability of the product and the reputation of the Australian industry as a supplier of quality product. Quality is becoming increasingly more important as Australian traders attempt to establish market share against other chickpea exporting countries such as Canada, Turkey and Mexico.
It is highly likely that we will see much greater segregation and premiums paid for lighter coloured, large seeded desi types as new varieties with these traits are developed and the Australian industry becomes more quality conscious.
Weathering of seed due to delays in harvesting can substantially increase mould infection levels. High levels of mould infection will also cause darkening of the seed coat. Humid (above 70% RH), wet conditions favour the development of a range of fungi in late harvested chickpea crops.
Ascochyta can develop on dry senescing pods under wet conditions, and infection can penetrate through to the seed. The current Export Receival Standard for visible ascochyta lesions is a maximum of 1% on the seed cotyledon (kernel).
Native budworm larvae can occasionally attack senescing chickpeas, particularly where rainfall has softened the pod. Insect damaged seeds are classified as Defective chickpeas.
Early harvest gives the grower some degree of control over how and when the crop is marketed, whereas the late harvested chickpeas can be price takers in a falling market.
Implementing early harvest management
There are a range of management components that contribute to an early crop. Use the strategies below, according to the conditions of the season, to achieve early harvest and optimal yield, quality and profit.
Planting
- Sow at the earliest opportunity within the preferred planting window for your area. Moisture seeking equipment and/or press wheels can significantly enhance seeding opportunities under marginal conditions.
- Select adapted varieties that meet your target for early harvesting.
- Using precision planters will often achieve more uniform plant establishment and crop development, and consequently more even crop maturity.
In-crop management
- Control of Botrytis grey mould if present during flowering.
- Control of native budworm during flowering to maximise early pod set.
- Avoid using herbicides that delay crop maturity i.e. flumetsulam (e.g. Broadstrike®).
Harvest management
- Consider using Roundup Power MAX® + Ally® (or equivalent registered products) to terminate crop at 80–90% yellow-brown pod stage.
- Set up header to operate efficiently at 14–15% grain moisture content.
High moisture harvesting can commence earlier in the season and earlier each day. Harvesting at 14% moisture content can effectively double the harvest period available on any one day, compared to harvesting at 12% moisture content. Blend, aerate and/or dry the sample to the required receival standard of 14% moisture.
Desiccation
In chickpeas, desiccation can occur when 80–85% of pods have turned from green to yellow-brown and 90% of seed has begun to lighten in colour (indicating physiological maturity).
Desiccation is a valuable management tool to assist with:
- green weeds present at harvest
- harvest efficiency—eliminating many of the problems associated with green stems and gum build-up that causes uneven flow of material through the header, which enables drum speeds to be reduced, reducing the proportion of cracked or damaged grain in the sample
- avoiding the risk of chickpeas re-shooting and re-flowering in response to 'early' summer rain
- patchy and or delayed crop maturity on heavy clay soils
- implementing ‘early harvest management’.
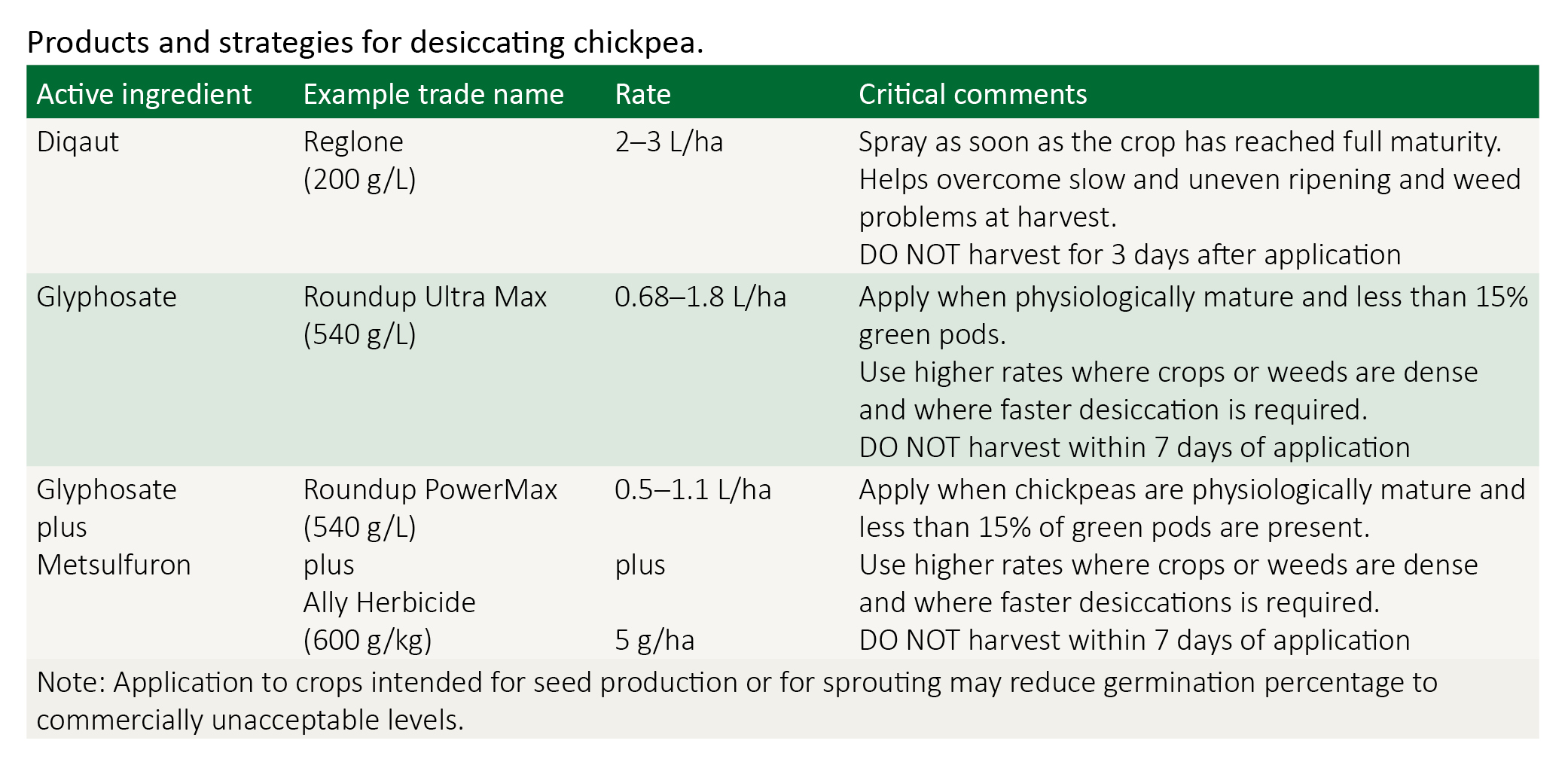
For more detailed information: Desiccation and croptopping in pulses
Harvesting
When harvesting for seed, take extra care to reduce grain cracking even if this means making a poor sample. Gentle harvesting will give the best seed quality. Rotary harvesters are gentler on the crop and will generally cause less grain damage than conventional harvesters.
The crop varies in height from 15–80 cm, with pods held up in the canopy, so direct heading without crop lifters is possible with open front and closed front machines. Open-front or pick-up fronts are best suited to the job. Some fingers may have to be removed when using closed-front machines. Chickpeas thresh easily but are prone to cracking, particularly kabuli types, so adjust thresher speed (400–600 rpm) and concave (10–30 mm) to suit. Removing alternate wires and blank-off plates from the concave will help reduce cracking. If possible cover the rasp bars with plate.
Harvested chickpea crops as soon as they mature as pods will fall if harvest is delayed. Harvesting grain at high moisture levels up to 14 per cent should minimise cracking. Early harvesting, before summer weeds become a problem, will reduce clogging and sample contamination. Desiccating the crop will kill summer weeds and ensure even crop ripening.
As chickpeas are destined for human consumption a good sample off the header is usually required.
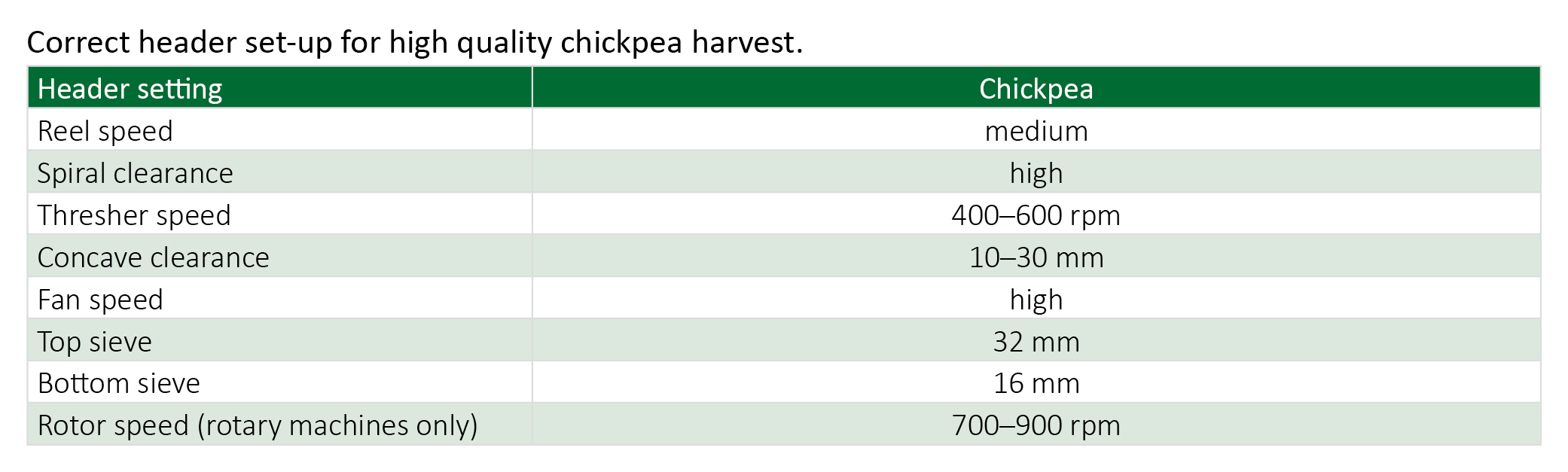
For more detailed information: Chickpea harvest and storage
Handling
Chickpeas have an irregular seed shape with an exposed beak, and are very prone to mechanical damage during handling. This especially applies to:
- overly dry grain (below 13% MC).
- crops that have been exposed to weather damage prior to harvest.
The use of tabulators or belt conveyors can reduce damage compared to conventional spiral augers.
Grain can be handled up to six times before delivery to receival points, so it is important that growers minimise the number of handling stages wherever possible, and use efficient handling techniques that minimise damage.
Grain storage
Growers contemplating medium to long term storage (6–12 months) need to be aware that chickpeas continue to age, and that quality deteriorates over time.
Desi chickpeas will darken considerably in storage, with the rate of seed coat darkening being accelerated by:
- high seed moisture content
- high temperatures
- high relative humidity.
Seed subject to field weathering prior to harvest will deteriorate a lot quicker in storage, even when stored under 'acceptable' conditions of temperature and relative humidity. Storing field weathered grain is not recommended.
To maintain yellow colour and minimise darkening of seed, any grain stored above 12% MC will require cooling.
Pulse grain placed in storage with high germination and vigour will remain viable for at least 3 years providing the moisture content of the grain does not exceed 11%. Storage life of pulses is determined by temperature, insects and diseases.
Insects are not considered a major problem in stored chickpeas except where chickpeas are loaded into storages containing residues of cereal grain already infested with rust-red flour beetle (Tribolium castaneum), lesser grain borer (Rhyzopertha dominica) or saw-toothed grain beetle (Oryzaephilus surinamensis). Where a prior infestation exists in the storage structure, insect numbers can increase and spread in the chickpeas.
The key to control is to ensure that all handling equipment and storages are cleaned of old cereal grain before they are used to handle chickpeas. Good hygiene combined with aeration cooling should prevent infestations developing. If insects are found in stored chickpeas, the only registered treatment is phosphine fumigation.
For more detailed information:
- Storing pulses in grain bags
- Pulse storage video (GRDC)
- Pulse storage factsheet (Stored Grain)
-
Marketing, enduses and standards
Chickpea was first produced in the Middle East about 7000 years ago and is now produced in over 50 countries and on all continents. The most important chickpea producing countries are India, Turkey, Pakistan, Iran, Mexico, Ethiopia, Myanmar, Canada and Australia.
India is the largest producer of chickpea, accounting for 66% of global production and 63% of cultivated area.
Chickpea is currently grown on approx. 12 million hectares worldwide with average annual production ranging from 8–10 million tonnes. On average, world production is approximately 75% desi and 25% kabuli types. About 95% of chickpea cultivation and consumption is in the developing countries. The top three exporting countries (Australia, India and Mexico) account for 65% of exports.
The main three importing countries India, Pakistan, and Bangladesh account for 61% imports with and additional 18% imported into the next three main importing countries of Spain, Algeria and United Arab Emirates.
International trade in chickpea is negligible compared to other agricultural commodities. The marketed volume is only 5–8% of the total production as most chickpeas are consumed in the countries where they are produced. There is insufficient volume to support a transparent marketing system such as futures, which is available in the other grains such as corn, wheat and canola.
The price of chickpeas as a commodity is relatively stable, rising and falling in line with supply and demand. Traditionally there were stronger markets prior to the Ramadan festive season but that is not as relevant today.
The relatively small tonnage traded also means that any delay in harvest or shipping in an exporting country that threatens a contract being met can result in a ‘short’ or ‘spike’ in the market. These are normally short term and reflect a contractual supply problem rather than a fluctuation in the chickpea market.
Whilst ‘spikes’ and ‘shorts’ are hard to predict, the understanding of chickpea marketing is improving throughout chickpea growing areas in Australia with competition between local buyers, improved communication between growers and marketers, and more information being available through Pulse Australia market news and other sources.
In recent years the price of chickpeas has generally shown a rising trend as production in the traditional countries has fluctuated with often-reduced production for a number of reasons. This situation is connected with insufficient expansion in the importing countries to fill their domestic demand.
Marketing desi chickpea
Growers have a range of buyers and types of contracts available. Common contract options, apart from selling after harvest for the price of the day include:
- Firm price / firm tonnage: There is also a delivery date or period. This contract normally offers the best price of the day but the grower is committed to deliver the nominated tonnage.
- Minimum price hectare contracts: These contracts offer the grower a minimum guaranteed price for the total production from the area contracted. There may be a maximum tonnage included in the contract. This contract offers the grower the security of a minimum price but does commit them to one buyer. Part or the entire contract can be priced at any time prior to delivery i.e. committed to FIXED PRICE / FIXED TONNAGE. These contracts are popular with growers.
Marketing kabuli chickpea
Kabuli chickpeas represent as much as 15% of the Australian chickpea crop area crop, but less than 5% in the northern region where most chickpeas are currently grown. They are however about two-thirds of the southern chickpea crop area.
Whereas desi chickpea are basically exported to predominantly to three countries (India, Bangledesh and Pakistan only), kabulis are exported to a many smaller market destinations.
Australian small kabulis have been traded internationally for several years now, and there is increasing market awareness and anticipated continuing demand. Sometimes the opportunities can be more limited or less ‘fluid’ in terms of timing, continuity and ability to supply when there is demand. Storage or warehousing might be required, and this has cash flow implications to the grower.
Contracts available for kabuli chickpea are the same as for desi with the addition of a closed loop marketing arrangement for the kabuli variety Bumper only. This type of marketing arrangement requires that the total production from the crop is delivered to the buyer. The company holding the rights is the only one that can supply the seed or contract the crop.
For more detailed information:
Global chickpea production calendar
Chickpea is grown in winter in Australia but is grown in both seasons in the Indian subcontinent, winter (rabi) and summer (kharif).
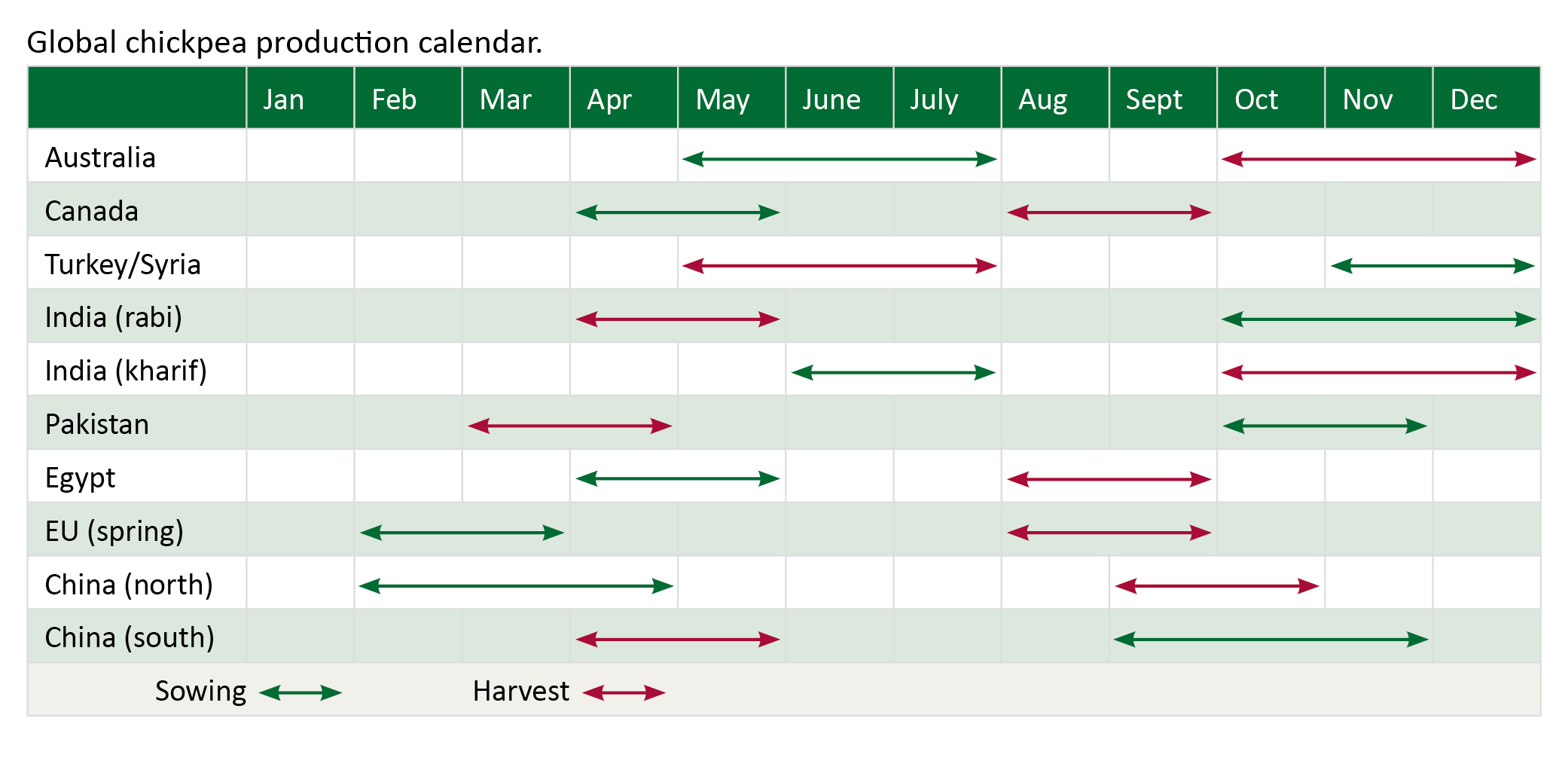
Building a relationship with a trusted chickpea buyer or marketer will usually be very worthwhile.
Receival standards
National receival standards for chickpea are set by the pulse industry and maintained by Pulse Australia. Receival and export standards reflect the market requirements for a quality food product. Desi type chickpeas should be sound, dry, fresh and light to medium brown in colour (a greenish tinge is allowed). Black is excluded as the predominating class. Kabuli type chickpeas should be sound, dry, fresh and cream to light brown in colour. Dark brown to black is excluded as the predominating class.
Sample desi chickpea visual quality chart and order form is available on the Receival and Trading Standards page.
Failure to achieve these receival standards may mean price discounts, re-cleaning or, if severe, market rejection.
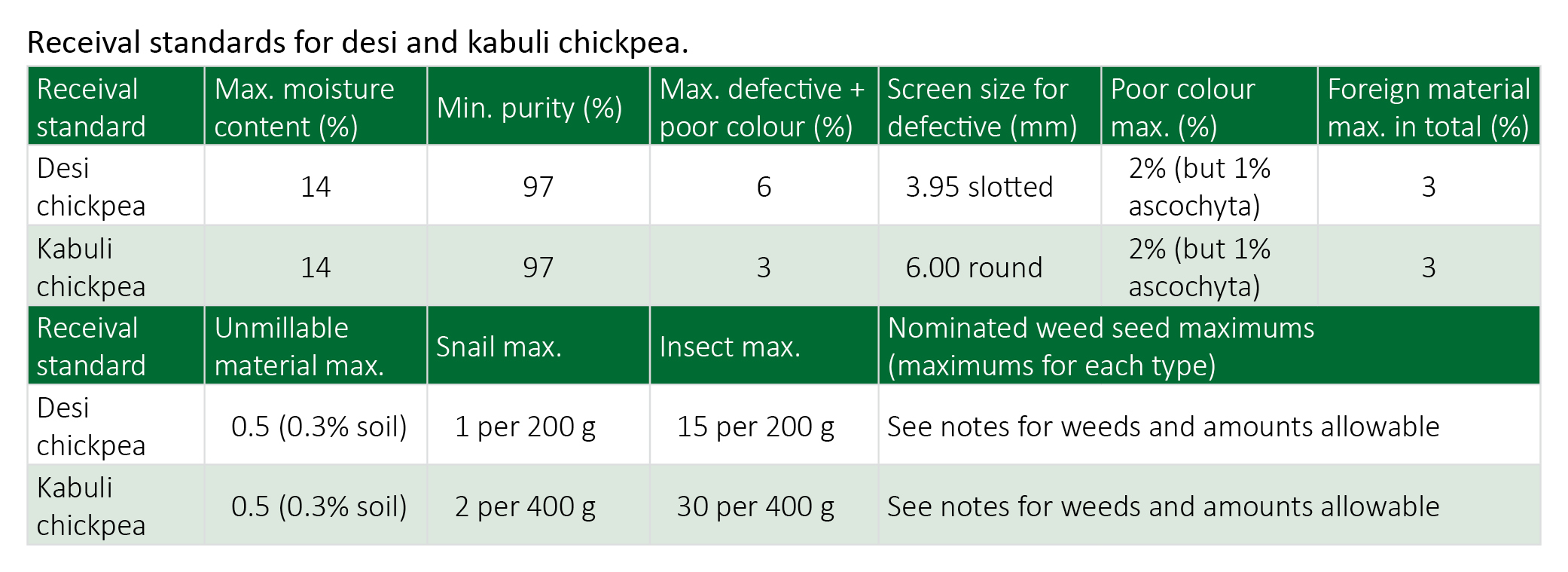
Definitions:
- Defective grains: includes max 2% field peas (in Desi), 2% poor coloured grains, broken, damaged and split, shrivelled, distorted, grub eaten, sprouted and affected by field mould.
- Poor colour: if cotyledon is distinctly blemished and/or off colour from the characteristic yellow colour of the predominate class, including the 1% visible ascochyta.
- Foreign material: includes unmillable material and all foreign vegetable matter (includes cereals, wild Oats, oilseeds, other legumes and weed seeds not otherwise specified).
- Unmillable material: includes soil, stones, metal and non-vegetable matter.
Nominated foreign weed examples
- Type 1 (4 per 200 g): three-cornered Jack
- Type 2: (nil per 200 g): wild garlic, coriander and any other tainting agents
- Type 3a (1 per 200 g in total): Bathurst burr, caltrop
- Type 3b (2 per 200 g total): vetches (tares)
- Type 3c (4 per 200 g total): heliotrope
- Type 4a (10 per 200 g total): cut leaf mignonette, melilotus (if no taint), nightshades, skeleton weed, variegated thistle
- Type 5 (20 per 200 g in total): knapweed, salvation Jane
- Type 6 (5 seeds or pods total per 200 g) medic pods, marshmallow pods, saffron thistle, wild radish pods
- Type 7a (10 seeds per 200 g total): other pulses
- Type 7b (10 seeds per 200 g total): cereals, turnip weed, bindweed
- Type 7c (1 seed in total per 200 g): safflower, sunflower
- Type 8 (100 seeds per 200 g): bellvine.
- Small foreign seeds (0.6% by weight): amsinkia, canola, charlock, marshmallow seeds, hedge mustard, etc
For more detailed information: 'Pulse receival and export standards'
Key contacts
Pulse Australia Industry Development Managers
- Paul McIntosh
Phone: 0429 566 198 - Phil Bowden
Phone: 0427 201 946
Support and funding acknowledgement
Disclaimer
Information provided in this guide was correct at the time of the date shown below. No responsibility is accepted by Pulse Australia for any commercial outcomes from the use of information contained in this guide.
The information herein has been obtained from sources considered reliable but its accuracy and completeness cannot be guaranteed. No liability or responsibility is accepted for any errors or for any negligence, omissions in the contents, default or lack of care for any loss or damage whatsoever that may arise from actions based on any material contained in this publication.
Readers who act on this information do so at their own risk.
Copyright © 2015 Pulse Australia
All rights reserved. The information provided in the publication may not be reproduced in part or in full, in any form whatsoever, without the prior written consent of Pulse Australia. www.pulseaus.com.au
Last updated: 15 January 2016



