Australian Pulse Bulletin
Chickpea: High quality seed
Leigh Jenkins1, Kevin Moore1, Gordon Cumming2
1NSW Department Primary Industries, 2Pulse Australia
Summary
- High quality seed is essential to ensure the best start for your crop.
- Grower retained seed may be of poor quality with reduced germination and vigour, as well as being infected with seed-borne pathogens.
- All seed should be tested for quality including germination and vigour.
- Consider purchasing seed from commercial suppliers, if grower retained seed is of low quality.
- Regardless of source, treat all seed with a thiram-based fungicide.
- Careful storage and handling is required for seed prior to sowing.
- Calculate seeding rates in accordance with seed quality (germination, vigour and seed size).
No matter whether chickpea seed is acquired commercially or grower retained on-farm, it is important that it is of the highest possible quality. Poor quality seed can result in low plant establishment due to poor germination, vigour and/or seed-borne diseases such as Ascochyta blight and Botrytis grey mould (BGM).
Chickpea growers can minimise further deterioration of seed quality with adequate planning and consideration of management options prior to sowing.
Grower retained seed
Seed quality may be adversely affected by several factors including:
- Early desiccation resulting in high levels of green immature seed and smaller seed size (affecting both germination and vigour).
- Cracking of the seed coat if the seed is exposed to several wetting and drying cycles. As the seed coat absorbs moisture it expands and then contracts as it dries. This weakens the coat increasing the risk of mechanical damage during harvesting and handling operations.
- Mechanical damage can result in reduced germination and vigour and increased susceptibility to fungal pathogens in the soil at sowing (exacerbated if establishment is delayed into cold wet soils).
- Delayed harvest due to wet weather can lead to increased (i) heliothis damage; (ii) mould infection, and (iii) risk of late ascochyta seed infection.
- Harvesting at a moisture content > 15% can lead to problems with moulds and fungal pathogens colonising on the seed coat during storage.
- Harvesting at a moisture content < 10% can result in mechanical damage to the seed coat and/or seed splitting, which is compounded each time the seed is handled.
- Poor (temporary) storage in the rush to get harvest done in wet weather can reduce viability of the seed resulting in poor germination and emergence.
- Using chickpea seed which was “shot and sprung” when harvested is not recommended. Chickpea is a relatively large seed which requires considerable moisture for germination to begin. Unlike cereal grains with higher starch content, once the chemical process has started and an embryo formed, the seed will continue to germinate, use up its energy reserves and die due to lack of follow-up moisture whilst in storage.
- Seed-borne diseases such as ascochyta blight and botrytis grey mould, and/or in contamination with sclerotinia, all reduce the viability of the seed (germination and vigour). Crop establishment is reduced and any surviving infected seedlings act as an inoculum source to initiate disease infection within the new crop.
Regardless of source, all seed should be treated with a registered fungicide seed dressing (see below).
Testing seed for quality
Grower retained seed should be tested for germination and vigour at least twice:
- Immediately post-harvest, to assess if the seed is worth retaining or better sold for grain or fed to livestock.
- Just before sowing, after grading and treatment with fungicide seed dressing, so seeding rates can be adjusted to compensate for any decline in quality during storage over summer.
Weather damaged seed deteriorates quicker than usual in storage and a third test in mid-storage is advised. This will indicate if the seed is still suitable for sowing and if not, allows time to consider other options.
Pulse seed should have a minimum germination of 80% to be kept for sowing. Seeding rates for seed with only 50% germination will need to be doubled. It is worth considering whether machinery can meter out such higher rates of seed.
Whilst increasing the seeding rate of poor quality seed may seem reasonable, it carries a high risk of seedling disease. If the poor quality is caused by seed-borne pathogens, the seed will be a source of infection for healthy seed and seedlings.
Simple germination tests can be done at home at any time over summer as temperature does not affect the results. There are two quick methods that both require 100 seeds for the test:
- Method 1: Place 100 seeds between at least four sheets of paper towel, roll up, fold the ends over and soak in fresh water for 30 minutes. Drain, put the 'seed doll' in a tray and place on the kitchen bench or workshop table. Ensure 'seed doll' does not dry out. After 3, 4 and 5 days unwrap 'doll', remove germinated seeds, taking a note of their vigour and re-wrap the 'doll'. After the fifth day count the non-germinated and mouldy seeds.
- Method 2: Fill a flat shallow (5 cm deep) garden tray or non-rusty baking tray with clean sand, potting mix or freely draining soil. On the soil surface arrange your 100 sends in 10 rows and push the seeds into the soil with a pencil marked to a depth of 3 cm. Cover with a little more sand/soil and water gently. Keep soil moist but not wet as overwatering will result in fungal growth and possible rotting.
Research on 2010 harvested seed demonstrated that a germination test does not always accurately predict emergence. Accordingly, growers are advised to conduct their own emergence test, as per method 2. If this is conducted in the intended paddock this will also help to identify potential herbicide residue problems.
After 7–10 days the majority of viable seeds will have emerged, count only the normal and healthy seedlings. If left for another few days you will also be able to assess how many of the germinated seeds are actually deformed (e.g. missing cotyledons or mould/disease affected), which will also give some indication of vigour.
-

Doing your own germination test. Photo: E. Leonard, AgriKnowHow
-

After 5 days (right) and 12 days (left). Photo: Kevin Moore
It is recommended that both the germination test and seed size test be done on several lots of seed (i.e. at least twice) to get a more accurate assessment of the sample.
Seed vigour is more accurately assessed by commercial laboratories as the assessment method is both temperature and time based. The seedling vigour test will also provide a better indication of the number of abnormal seeds present in addition to the germination percentage.
Seed testing laboratories
| Futari Grain Technology Services
PO Box 95 ph: 02 6792 4588 |
SGS Agritech
214 McDougall St ph: 07 4633 0599 |
Note: A minimum of 500 grams of seed is required for testing.
Handling seed on farm
To minimise physical damage to the seed:
- Plan farm operations so that handling is kept to a minimum.
- Use belt conveyors or brush flight augers, rather than spiral (screw) augers.
- Run augers slowly and at full capacity.
- Where possible, avoid handling seeds with low (< 10%) moisture content.
Grading
Chickpea seed for sowing should be professionally graded (preferably using a gravity grader) to provide a uniform seed lot and remove all small or split seeds, trash and weed seeds. A gravity grader is more efficient at removing lighter seeds, which are often disease infected. A higher proportion of small, shrivelled and light-weight grain can be expected in crops that have experienced high incidences of ascochyta and or botrytis grey mould.
Storage
Most pulse seed should only be stored for 12 months, although longer storage periods are possible with high quality seed provided both grain moisture and temperature within the silo can be controlled. Rapid deterioration of grain quality occurs under conditions of high temperature/moisture and with poor seed quality including weathered, cracked and diseased seed.
Ideally, chickpea needs to be stored at 13% moisture content and at temperatures below 30°C. Storage at very low moisture contents (< 10%) may make chickpeas (particularly kabuli types) more susceptible to damage during subsequent handling, as the seed shrinks away from the seed coat.
Reducing temperature in storage facilities (to below 30°C) is the easiest method of increasing seed longevity. Temperatures can be reduced in grain silos by painting the outside of the silo white (temperatures reduced by 4–5°C), and/or aerating silos with dry, ambient air.
Heat drying of chickpea seed should be limited to temperatures below 40°C.
Insect pests in storage
Insects are generally not considered to be a major problem in stored chickpea seed. However, where a prior infestation exists in the storage structure (most commonly as a result of cereal grain residue) then the infestation can develop and spread in the chickpeas. Ensure all handling equipment and storages are cleaned of old cereal grain before they are used to handle chickpeas. Good hygiene, combined with aeration cooling, should prevent infestations developing.
If insect pests are found in stored chickpeas, the only registered treatment is phosphine fumigation.
For more detailed information: Storing pulses (Stored Grain Hub)
Care at sowing
Effect of poor quality seed on yield
Using severely weather damaged or mechanically damaged seed will result in:
- Poor establishment and poor crop performance.
- Reduced plant vigour (increased susceptibility to soil-borne pathogens during establishment and increased susceptibility to foliar pathogens).
- Patchy, uneven plant stands (increased susceptibility to weed competition, aphids and viruses).
- Uneven plant development complicating in-crop management (e.g. herbicide applications).
- Uneven and delayed crop maturity (e.g. making desiccation timing difficult and leading to a mixed grain sample).
- Lower yields from a combination of all of the above.
At sowing time, seed quality can impact on future management decisions such as sowing time, sowing depth and seeding rate.
As a general guide, weather damaged seed with lower than normal germination and vigour levels should only be sown under very good conditions (for rapid establishment) and at a higher seeding rate. Such seed is not recommended for deep sowing or moisture seeking.
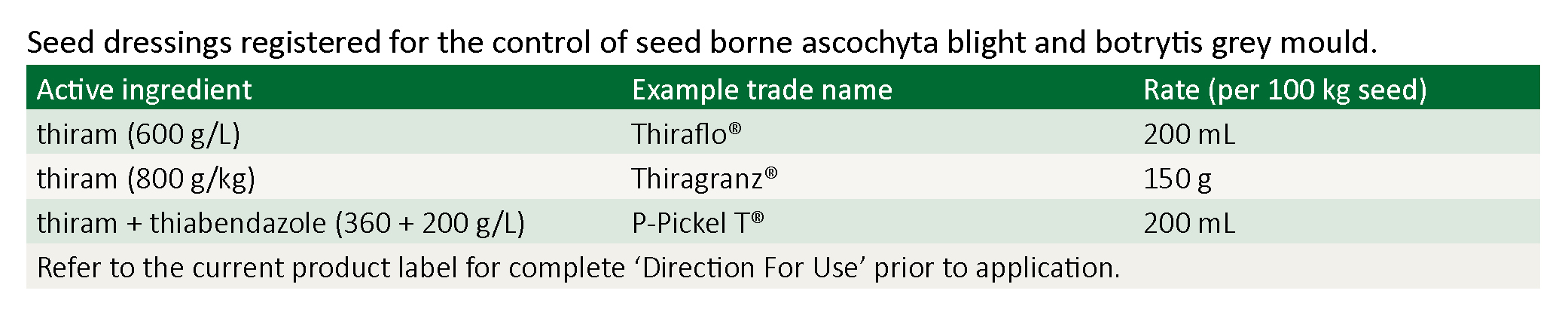
Seed dressings (pickling)
It is recommended that whenever possible seed should be obtained from a source where the crop was free from ascochyta blight and botrytis grey mould.
All seed, regardless of source, should be treated with a registered thiram-based fungicide seed dressing prior to sowing.
These seed treatments will help to minimise the levels of seed-borne ascochyta and botrytis grey mould. Research has shown that thiram plus thiabendazole products (e.g. P-Pickle-T®) and thiram only products (e.g. Thiraflo®) are equally effective against ascochyta and botrytis.
Botrytis grey mould (caused by the fungus Botrytis cinerea) not only causes the stem disease but also a seed-borne 'damping-off' disease of seedlings.
Infected seedlings will often emerge but die within a few weeks, resulting in significant establishment losses. Spores of B. cinerea are normally only present on the outside of mildly infected seed and control is obtained with seed dressing fungicides.
There are no known fungicide seed dressings or treatments to control sclerotinia, although grading may assist by physically reducing the number of small sclerotes (fungal fruiting bodies) in the seed sample.
Kabuli chickpeas may show a response to the application of fungicide seed dressings even in the absence of known fungal diseases. This is because kabulis have a thinner seed coat than desi types and a lower content of phenolic compounds, which help protect the seed against fungal attack.
Interaction with inoculation
All chickpea seed should be inoculated with Group N inoculant. Fungicide seed dressings can reduce the longevity of this nitrogen fixing bacteria. But if contact is kept to a minimum duration, satisfactory nodulation will be obtained in most cases.
Fungicide seed dressings can be applied at any convenient time leading up to sowing. However, inoculation of seed needs to occur as close as possible to the time of sowing.
All inoculated seed should be sown into moist soil within 12 hours of treatment (or as per the inoculant’s label directions), and the sooner the better. If inoculated seed is not sown within 12 hours, re-inoculate before sowing.
For more detailed information: Inoculating pulses
Sowing depth
It is generally recommended that chickpea seed should be sown 5–7 cm deep into moist soil. The preferred depth when using Balance® or simazine herbicides is 7 cm.
Sowing poor quality seed too deeply, into cold and/or wet soils, hard setting (crusting) soils and with some PSPE herbicides can reduce the ability of the germinating seedling to quickly reach the soil surface, which increases the seedling’s susceptibility to both soil and seed-borne pathogens, and soil-borne insect pests.
Moisture seeking strategies (i.e. planting at a depth of 10–15 cm below the soil surface) should be avoided for all weather damaged or low vigour seed.
Target plant density
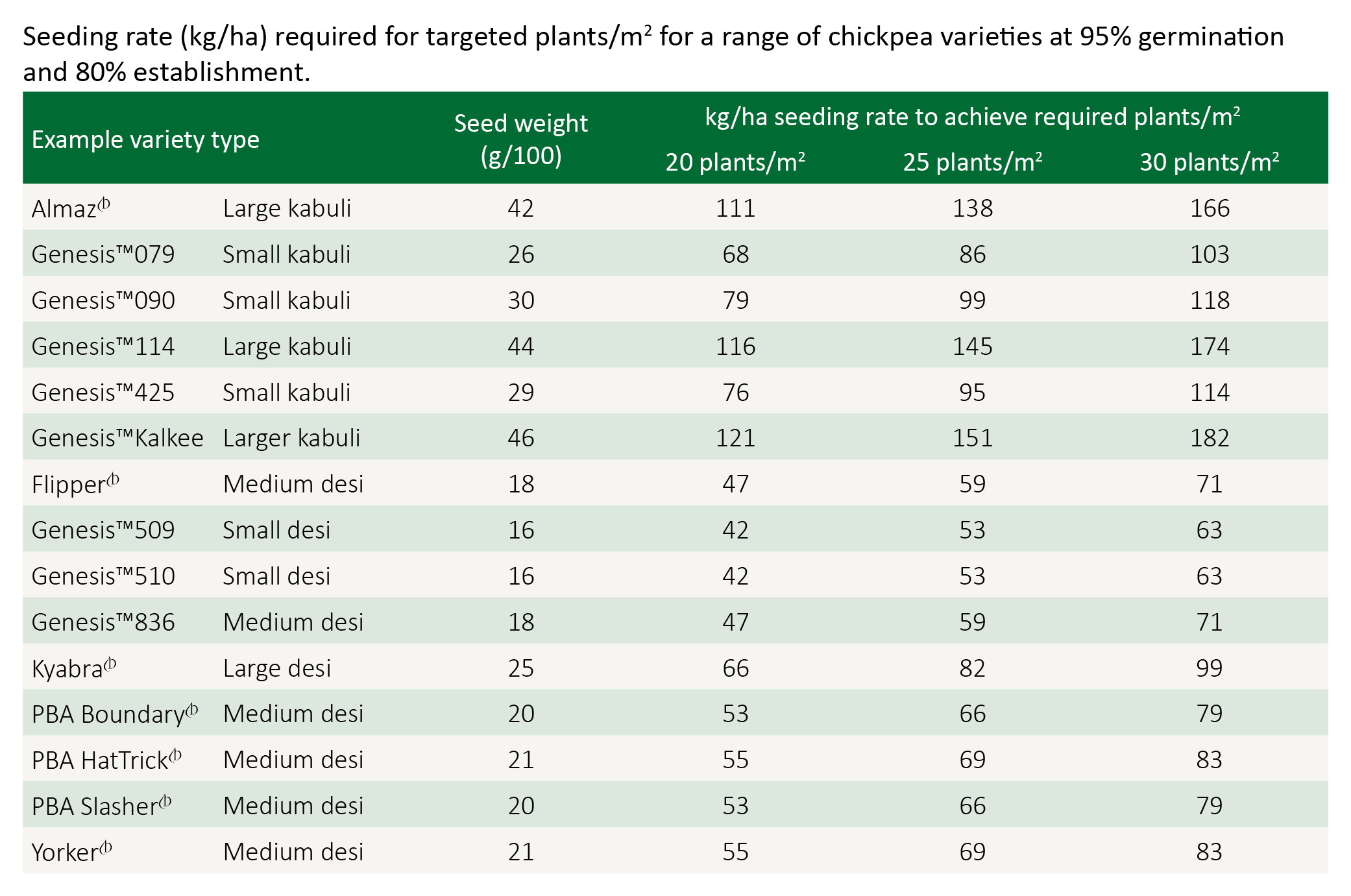
Under most conditions in central and northern NSW and southern and central Qld, aim to establish 20–30 plants/m2.
Adjust sowing rates to take account of seed size, germination percentage and estimated establishment conditions.
Higher populations are justified for late sowings, while lower populations of around 20 plants/m2 are often recommended for crops grown on wide row spacing (e.g. 100 cm). High populations sown on wide rows often result in thin main stems and a higher risk of lodging.
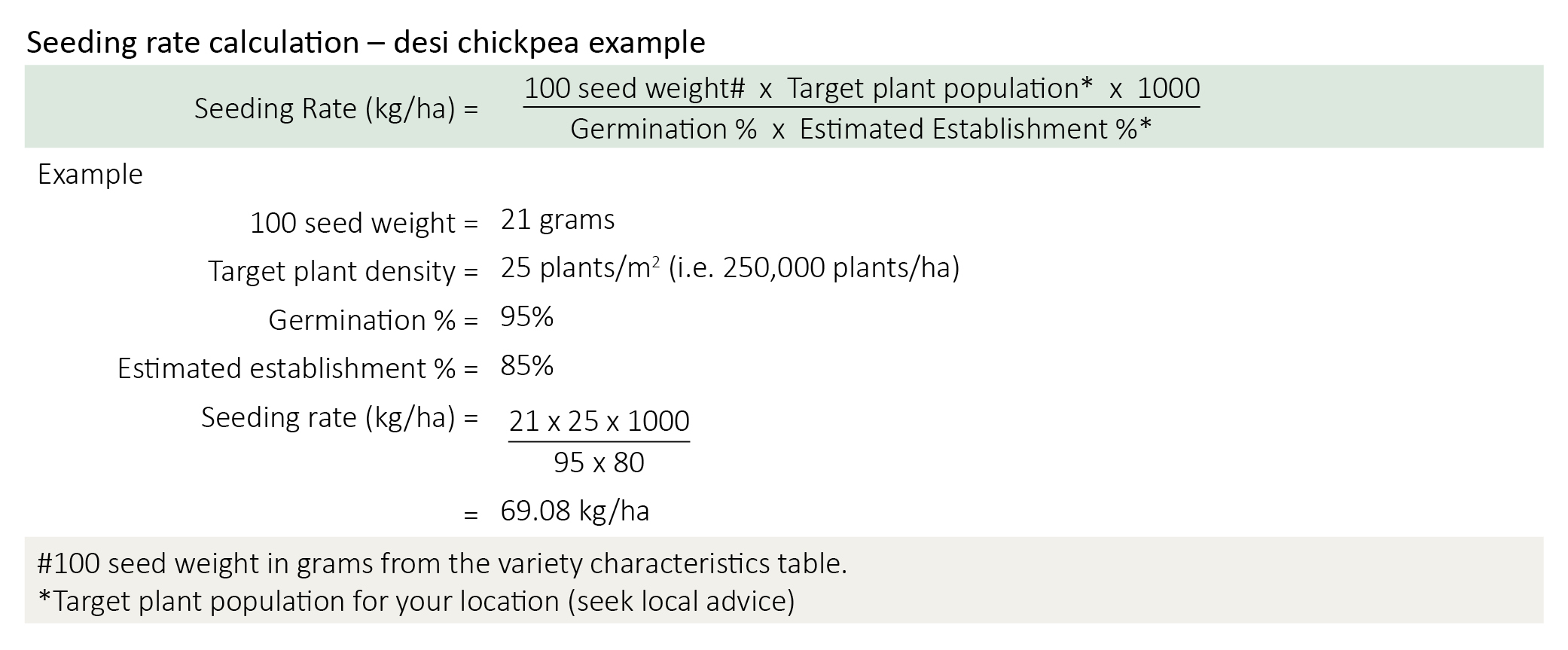
Calculating seeding rates
Seeding rate for the target plant density can be calculated using germination percentage, 100 seed weight and establishment percentage.
Commercial seed supplies
There is a limited amount of commercially grown seed available from the seed companies each year. Growers and agronomists are advised to determine their needs and communicate with suppliers at the earliest possible time to help secure their requirements. This also provides time for the industry to move seed stocks if possible to meet grower needs.
All commercially available seed lots must meet minimum quality standards as set by the variety owner.
Plant breeder’s rights
All currently recommended chickpea varieties for northern NSW are protected by Plant breeder’s rights (PBR) legislation.
As such seed produced by growers can only be retained for their own use. It cannot be sold, traded or bartered to other growers under any circumstances.
If grower retained seed is not suitable for sowing, replacement seed can be purchased through commercial suppliers. Growers are encouraged to sow only high quality seed as losses due to poor establishment (and possible re-sowing leading to further delayed establishment) are far greater than any savings gained in retaining poor quality seed.
Key contacts
Disclaimer
Information provided in this guide was correct at the time of the date shown below. No responsibility is accepted by Pulse Australia for any commercial outcomes from the use of information contained in this guide.
The information herein has been obtained from sources considered reliable but its accuracy and completeness cannot be guaranteed. No liability or responsibility is accepted for any errors or for any negligence, omissions in the contents, default or lack of care for any loss or damage whatsoever that may arise from actions based on any material contained in this publication.
Readers who act on this information do so at their own risk.
Copyright © 2015 Pulse Australia
All rights reserved. The information provided in the publication may not be reproduced in part or in full, in any form whatsoever, without the prior written consent of Pulse Australia.
Last updated: 20 November 2015
